ORGANIC CHEMISTRY
The Chemistry of Life
The Chemistry of Cells
NOTES - THE CHEMICAL COMPOSITION OF CELLS
CLICK ON THE SLIDE SHOW BELOW TO CONTROL THE SHOW!
PAUSE, PLAY, NAVIGATE FORWARD AND BACK AND SKIP AROUND!
PAUSE, PLAY, NAVIGATE FORWARD AND BACK AND SKIP AROUND!
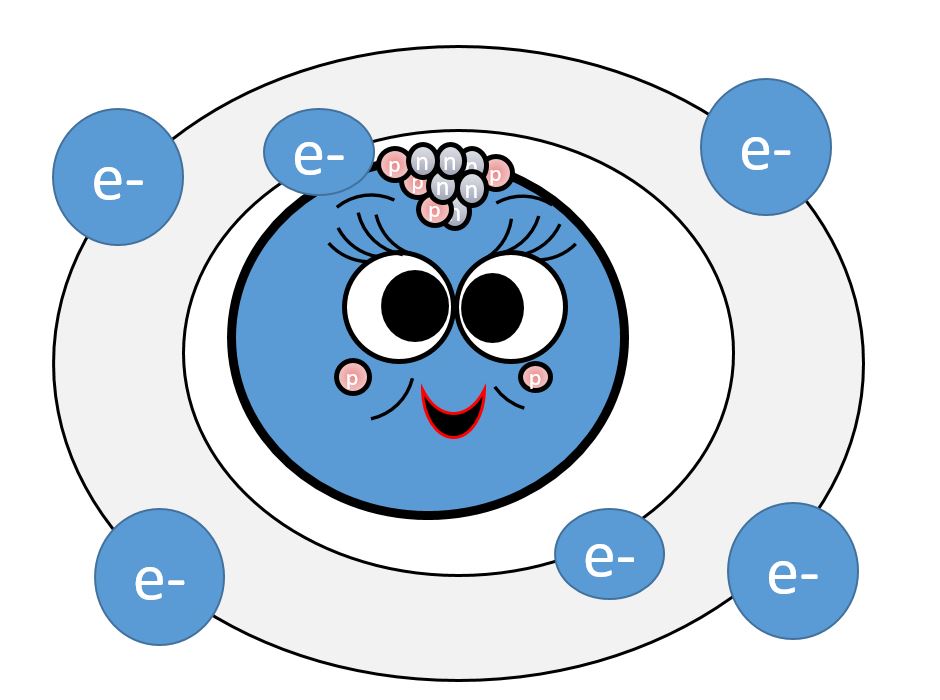
MEET CARMEN THE CARBON!!!
Introduction to Organic Chemistry
Life’s Diversity is due to being carbon-based. Most of the molecules in cell makes are made up of carbon atoms bonded to one another and to atoms of other elements. Carbon is able to form large and complex molecules, which build the structures and carry out the functions required for life. Carbon-based molecules are called organic compounds, and they usually contain hydrogen atoms as well.
Let's first look at the unique properties of Carbon that give rise to the vast majority of molecules necessary for life. The number of electrons in the outermost shell determines an atom’s chemical properties. A carbon atom has 4 electrons in a valence shell that holds 8. Carbon completes its outer shell by sharing electrons with other atoms in four covalent bonds. This allows for a huge variety of possibilities and possible structures. This is why we observe large organic molecules with complex and elaborate shapes.
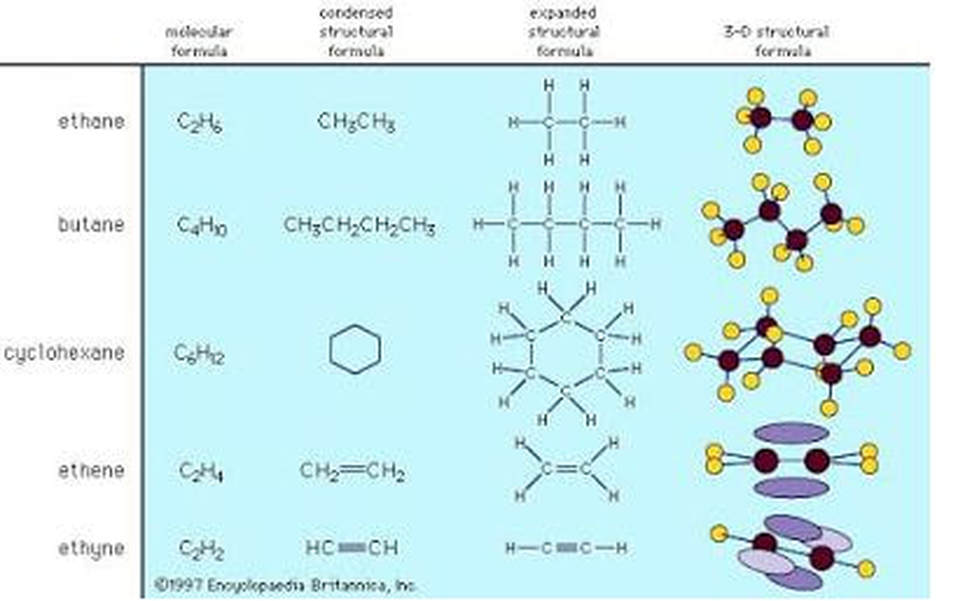
The shape of the organic molecule (and any molecule for that matter,) determines its function. Carbon chains form the backbone of most organic molecules. The diagram at the end of this section illustrates some of the possible shapes organic molecules can take. These molecules can differ in length and angle. For example, they can be straight, branched, or arranged in rings. Carbon skeletons may also include double bonds, which can vary in number.
ELECTRONEGATIVITY = Electronegativity is the tendency of an atom to attract electrons towards itself.
We know from C position on the periodic table, that it is in group 4. all group 4 elements react in such a way that they can form up to 4 bonds. The atomic number of C is 6. The first shell can hold up to 2 electrons. The second shell can hold up to 8 electrons. Atoms have a tendency to ionize in such a way that their outer-most shell is filled. They can do this either by gaining or losing electrons, but it will always take the “path of least resistance”.
Hydrocarbons
Hydrocarbons are composed of carbon and hydrogen. In nature, hydrocarbons take the form of crude oil and natural gas. That being said, hydrocarbons are the primary energy source for most of the world today. Hydrocarbons can be found in living organisms in the form of hydrocarbon chains within certain molecular structures. A good example of this are the hydrocarbon chains found in lipids (fats) that provide fuel to your body. Hydrocarbons are very good at forming covalent bonds with other hydrocarbons to form long chains or rings. There are two types of covalent bonding; polar and non-polar. In any covalent bond, electrons are SHARED between the bonded atoms. In a polar covalent bond, these electrons are unequally shared and they spend more of their time towards one of the bonded atoms than the other. This results in a "polar" molecule which will carry a partial negative near the atom with the higher electronegativity value (and, thus, the electrons preferentially will be located by that atom more often) and a partial positive charge at one end in which the electrons spend less time. In hydrocarbon chains, H and C have very similar electronegativties and from non-polar covalent bonds, and when C binds to another C they share their electrons completely equally since they have the same electronegativity value. We consider any bond between two elements with the same or similar electronegativities (within ~ 0.30) a non-polar bond. Hydrocarbons are considered hydrophobic meaning they are “afraid” of water! Hydrocarbons are the world's leading source of electrical and thermal energy, due to the amount of energy produced when burnt. This burning is a combustion reaction in which oxygen from the air becomes a reactant with the hydrocarbon, to form a new chemical product. Common products of hydrocarbons combustion reactions include steam, carbon dioxide and heat.
Life’s Diversity is due to being carbon-based. Most of the molecules in cell makes are made up of carbon atoms bonded to one another and to atoms of other elements. Carbon is able to form large and complex molecules, which build the structures and carry out the functions required for life. Carbon-based molecules are called organic compounds, and they usually contain hydrogen atoms as well.
Let's first look at the unique properties of Carbon that give rise to the vast majority of molecules necessary for life. The number of electrons in the outermost shell determines an atom’s chemical properties. A carbon atom has 4 electrons in a valence shell that holds 8. Carbon completes its outer shell by sharing electrons with other atoms in four covalent bonds. This allows for a huge variety of possibilities and possible structures. This is why we observe large organic molecules with complex and elaborate shapes.
The shape of the organic molecule (and any molecule for that matter,) determines its function. Carbon chains form the backbone of most organic molecules. The diagram at the end of this section illustrates some of the possible shapes organic molecules can take. These molecules can differ in length and angle. For example, they can be straight, branched, or arranged in rings. Carbon skeletons may also include double bonds, which can vary in number.
ELECTRONEGATIVITY = Electronegativity is the tendency of an atom to attract electrons towards itself.
We know from C position on the periodic table, that it is in group 4. all group 4 elements react in such a way that they can form up to 4 bonds. The atomic number of C is 6. The first shell can hold up to 2 electrons. The second shell can hold up to 8 electrons. Atoms have a tendency to ionize in such a way that their outer-most shell is filled. They can do this either by gaining or losing electrons, but it will always take the “path of least resistance”.
Hydrocarbons
Hydrocarbons are composed of carbon and hydrogen. In nature, hydrocarbons take the form of crude oil and natural gas. That being said, hydrocarbons are the primary energy source for most of the world today. Hydrocarbons can be found in living organisms in the form of hydrocarbon chains within certain molecular structures. A good example of this are the hydrocarbon chains found in lipids (fats) that provide fuel to your body. Hydrocarbons are very good at forming covalent bonds with other hydrocarbons to form long chains or rings. There are two types of covalent bonding; polar and non-polar. In any covalent bond, electrons are SHARED between the bonded atoms. In a polar covalent bond, these electrons are unequally shared and they spend more of their time towards one of the bonded atoms than the other. This results in a "polar" molecule which will carry a partial negative near the atom with the higher electronegativity value (and, thus, the electrons preferentially will be located by that atom more often) and a partial positive charge at one end in which the electrons spend less time. In hydrocarbon chains, H and C have very similar electronegativties and from non-polar covalent bonds, and when C binds to another C they share their electrons completely equally since they have the same electronegativity value. We consider any bond between two elements with the same or similar electronegativities (within ~ 0.30) a non-polar bond. Hydrocarbons are considered hydrophobic meaning they are “afraid” of water! Hydrocarbons are the world's leading source of electrical and thermal energy, due to the amount of energy produced when burnt. This burning is a combustion reaction in which oxygen from the air becomes a reactant with the hydrocarbon, to form a new chemical product. Common products of hydrocarbons combustion reactions include steam, carbon dioxide and heat.
Organic compounds are those that have carbon atoms. Remember carbon? It has 4 Valence electrons = 4 electrons in the outer shell = 4 electrons are available for bonding. Thus, carbon is able to make up to 4 strong bonds. carbon-based molecules can form a wide variety of shapes and long chains. Carbon is the element that we believe is necessary for LIFE.
Carbon Bonding
Carbon has 4 electrons available to form bonds with other atoms. This is why you will always see four lines connecting a carbon atom to other atoms. Each line represents a BOND that is formed by a pair of shared electrons (one electron from carbon and one from another atom). Each line represent a bond made with 1 valence electron from each atom participating in the bond. For example, methane (CH4) is a small organic molecule made up of one Carbon atom bound to 4 Hydrogen atoms.
CHEMICAL EVOLUTION
The first organic molecules were small . they were carbon-based molecules made up of only a few atoms. These small carbon-based molecules evolved to combined with other simple molecules to form more complex molecules. Over many years and probably trillions and trillions of chemical reactions, more complex molecules, and more stable molecules, formed.
All living things consist of organic molecules, centered around the element carbon. Organic molecules evolved before cells, perhaps as long as 4 billion years ago. Complex molecules can be formed by stringing carbon atoms together in a straight line or by connecting carbons together to form rings. The presence of nitrogen, oxygen, and other atoms adds variety to these carbon molecules.
C H O N = 96% of living things
How did these carbon-based molecules necessary for life first form? The first organic molecules formed about 4 billion years ago. Scientists believe lightening sparked chemical reactions in Earth’s early atmosphere. The early atmosphere contained gases such as ammonia, methane, water vapor, and carbon dioxide. Paleolightning is the study of lightning activity throughout Earth's history. Some studies have speculated that lightning activity played a crucial role in the development of not only Earth's early atmosphere, but also early life. Lightning, a non-biological process, has been found to produce biologically useful material through the oxidation and reduction of inorganic matter. Scientists hypothesize that this created a “soup” of organic molecules from inorganic chemicals. In 1953, scientists Stanley Miller and Harold Urey used their imaginations to test this hypothesis. They used a mixture of gases to represent Earth’s early atmosphere. Then, they passed sparks through the gases to represent lightning. Within a week, several simple organic molecules had formed.
RNA World Hypothesis
RNA may have been the first organic molecule to form as well as the basis of early life.
Primitive cells
The first cells consisted of little more than an organic molecule such as RNA inside a lipid membrane.
One cell (or group of cells), called the last universal common ancestor (LUCA), gave rise to all subsequent life on Earth.
Photosynthesis evolved by 3 billion years ago and released oxygen into the atmosphere.
Cellular respiration evolved after that to make use of the oxygen.
Complex molecules can be formed by stringing carbon atoms together in a straight line or by connecting carbons together to form rings.
The presence of nitrogen, oxygen, and other atoms adds variety to these carbon molecules.
Complex molecules can be formed by stringing carbon atoms together in a straight line or by connecting carbons together to form rings. The presence of nitrogen, oxygen, and other atoms adds variety to these carbon molecules.
MAKE SURE YOU KNOW THESE!!
Four important classes of organic molecules
Carbohydrates (sugars)
Lipids (fats)
proteins
nucleic acids ( RNA and DNA )
Carbohydrates
Sugar molecules have the formula (CH2O) n , where n is any number from 3 to 8. For glucose, n is 6, and its formula is C6H12O6. The formula for fructose is also C6H12O6, but as you can see in Figure 1, the placement of the carbon atoms is different.
Carbohydrates are classified into three groups according to the number of sugar (or saccharide) molecules present:
1) monosaccharides ( mono = 1, saccharide = sugar )
2) disaccharides ( di = 2, saccharide = sugar )
3) polysaccharides ( poly = many, saccharide = sugar )
Artificial Sugars
Aspartame is a common sugar substitute used in brands like Equal, and Nutrasweet, as well as diet soft drinks and chewing gums. Aspartame is not a sugar at all. It is a dipeptide (two amino acids joined together, as in a protein). But it's is a very slightly modified dipeptide.
180 times as sweet as sugar (sucrose). In this case, the artificial sweetener sucralose looks very similar to the natural sugar sucrose.
Sucralose is in some soft drinks and is marketed as an alternative to aspartame.
What is high-fructose corn syrup (HFCS)? Let’s start with the corn syrup part. The main carbohydrate in corn is starch, a polysaccharide built from glucose monomers. Industrial processing hydrolyzes starch into these monomers, producing corn syrup. Glucose, however, does not taste as sweet to us as sucrose. Fructose, on the other hand, tastes much sweeter than both glucose and sucrose. When a new process was developed in the 1970s that used an enzyme to rearrange the atoms of glucose into the sweeter isomer, fructose, the high-fructose corn syrup industry was born. (High-fructose corn syrup is a bit of a misnomer, because the fructose is combined with regular corn syrup to produce a mixture of about 55% fructose and 45% glucose, not much different from the proportions in sucrose.)
MAYO CLINIC - Research has shown that high-fructose corn syrup is chemically similar to table sugar. Controversy exists, however, about whether the body handles high-fructose corn syrup differently than table sugar. At this time, there's insufficient evidence to say that high-fructose corn syrup is any less healthy than other types of sweeteners. It is known, however, that too much added sugar of all kinds — not just high-fructose corn syrup — can contribute unwanted calories that are linked to health problems, such as weight gain, type 2 diabetes, metabolic syndrome and high triglyceride levels. All of these boost your risk of heart disease.
Lipids
Lipids are a class of substances that are insoluble in water. They are hydrophobic (water-fearing).
There are three major groups of lipids:
1) Triglycerides - fats, oils, and waxes.
2) Phospholipids – makes up cell membranes. Will spontaneously form a membrane in water in the lab!
3) Steroids - hormones
Triglycerides consist of three fatty acids bonded to a glycerol molecule. Fatty acids are hydrocarbons (chains of covalently bonded carbons and hydrogens) with a carboxyl group (–COOH) at one end of the chain. A saturated fatty acid the maximum possible number of hydrogens bonded to it. The carbon back bone contains only single bonds. Unsaturated fatty acids have less hydrogen bonded to it due to having one or more double bonds. This double bond is a the CIS configuration. “Transfats” are unsaturated fats that have one or more double bonds in a TRANS configuration. [CIS (same) vs TRANS (opposite)] CIS fats are healthy fats that promote good cholesterol. TRANS fats, on the other hand, can be harmful and can contribute to a decrease in cardiovascular health. Examples of TRANS fats include substances that are made from unnatural sources like hydrogenated oils. In November 2013, the U.S. Food and Drug Administration (FDA) put forth a mandate that food companies eliminate TRANS fats from their products over time.
Phospholipids are the major component of cell membranes. Phospholipids are structurally similar to fats, except that they contain only two fatty acids attached to glycerol instead of three. they consist of a hydrophillic (polar) head and 2 hydrophobic (non-polar) tails.
Amphiphilic.
Phospholipids
Phospholipids spontaneously form a double-lipid bilayer (like the cell membrane) in water.
Chemistry may have brought forth “life”.
Steroids
PROTEINS
Nearly every dynamic function in your body depends on proteins.
A protein is a polymer of small building blocks called amino acids.
Of all of life’s molecules, proteins are structurally and functionally the most elaborate and varied.
Proteins
Proteins are polymers of amino acids.
Proteins perform a vast array of functions within organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another.
Making Polymers - dehydration reaction
One monomer loses a hydroxyl group (OH-) and the other monomer loses a hydrogen (H) atom to form H2O.
As this occurs, a new covalent bond forms, linking the two monomers.
Breaking Polymers
HYDROLYSIS
“hydro” = water
“lysis” = to cut
Cells not only make macromolecules but also have to break them down.
Most of the organic molecules in your food are in the form of polymers that are much too large to enter your cells.
Nucleic Acids
DNA and RNA are built from single units (monomers) of nucleic acids.
DNA = Deoxyribonucleic acid
RNA = Ribonucleic acid
Carbon Bonding
Carbon has 4 electrons available to form bonds with other atoms. This is why you will always see four lines connecting a carbon atom to other atoms. Each line represents a BOND that is formed by a pair of shared electrons (one electron from carbon and one from another atom). Each line represent a bond made with 1 valence electron from each atom participating in the bond. For example, methane (CH4) is a small organic molecule made up of one Carbon atom bound to 4 Hydrogen atoms.
CHEMICAL EVOLUTION
The first organic molecules were small . they were carbon-based molecules made up of only a few atoms. These small carbon-based molecules evolved to combined with other simple molecules to form more complex molecules. Over many years and probably trillions and trillions of chemical reactions, more complex molecules, and more stable molecules, formed.
All living things consist of organic molecules, centered around the element carbon. Organic molecules evolved before cells, perhaps as long as 4 billion years ago. Complex molecules can be formed by stringing carbon atoms together in a straight line or by connecting carbons together to form rings. The presence of nitrogen, oxygen, and other atoms adds variety to these carbon molecules.
C H O N = 96% of living things
How did these carbon-based molecules necessary for life first form? The first organic molecules formed about 4 billion years ago. Scientists believe lightening sparked chemical reactions in Earth’s early atmosphere. The early atmosphere contained gases such as ammonia, methane, water vapor, and carbon dioxide. Paleolightning is the study of lightning activity throughout Earth's history. Some studies have speculated that lightning activity played a crucial role in the development of not only Earth's early atmosphere, but also early life. Lightning, a non-biological process, has been found to produce biologically useful material through the oxidation and reduction of inorganic matter. Scientists hypothesize that this created a “soup” of organic molecules from inorganic chemicals. In 1953, scientists Stanley Miller and Harold Urey used their imaginations to test this hypothesis. They used a mixture of gases to represent Earth’s early atmosphere. Then, they passed sparks through the gases to represent lightning. Within a week, several simple organic molecules had formed.
RNA World Hypothesis
RNA may have been the first organic molecule to form as well as the basis of early life.
Primitive cells
The first cells consisted of little more than an organic molecule such as RNA inside a lipid membrane.
One cell (or group of cells), called the last universal common ancestor (LUCA), gave rise to all subsequent life on Earth.
Photosynthesis evolved by 3 billion years ago and released oxygen into the atmosphere.
Cellular respiration evolved after that to make use of the oxygen.
Complex molecules can be formed by stringing carbon atoms together in a straight line or by connecting carbons together to form rings.
The presence of nitrogen, oxygen, and other atoms adds variety to these carbon molecules.
Complex molecules can be formed by stringing carbon atoms together in a straight line or by connecting carbons together to form rings. The presence of nitrogen, oxygen, and other atoms adds variety to these carbon molecules.
MAKE SURE YOU KNOW THESE!!
Four important classes of organic molecules
Carbohydrates (sugars)
Lipids (fats)
proteins
nucleic acids ( RNA and DNA )
Carbohydrates
Sugar molecules have the formula (CH2O) n , where n is any number from 3 to 8. For glucose, n is 6, and its formula is C6H12O6. The formula for fructose is also C6H12O6, but as you can see in Figure 1, the placement of the carbon atoms is different.
Carbohydrates are classified into three groups according to the number of sugar (or saccharide) molecules present:
1) monosaccharides ( mono = 1, saccharide = sugar )
2) disaccharides ( di = 2, saccharide = sugar )
3) polysaccharides ( poly = many, saccharide = sugar )
Artificial Sugars
Aspartame is a common sugar substitute used in brands like Equal, and Nutrasweet, as well as diet soft drinks and chewing gums. Aspartame is not a sugar at all. It is a dipeptide (two amino acids joined together, as in a protein). But it's is a very slightly modified dipeptide.
180 times as sweet as sugar (sucrose). In this case, the artificial sweetener sucralose looks very similar to the natural sugar sucrose.
Sucralose is in some soft drinks and is marketed as an alternative to aspartame.
What is high-fructose corn syrup (HFCS)? Let’s start with the corn syrup part. The main carbohydrate in corn is starch, a polysaccharide built from glucose monomers. Industrial processing hydrolyzes starch into these monomers, producing corn syrup. Glucose, however, does not taste as sweet to us as sucrose. Fructose, on the other hand, tastes much sweeter than both glucose and sucrose. When a new process was developed in the 1970s that used an enzyme to rearrange the atoms of glucose into the sweeter isomer, fructose, the high-fructose corn syrup industry was born. (High-fructose corn syrup is a bit of a misnomer, because the fructose is combined with regular corn syrup to produce a mixture of about 55% fructose and 45% glucose, not much different from the proportions in sucrose.)
MAYO CLINIC - Research has shown that high-fructose corn syrup is chemically similar to table sugar. Controversy exists, however, about whether the body handles high-fructose corn syrup differently than table sugar. At this time, there's insufficient evidence to say that high-fructose corn syrup is any less healthy than other types of sweeteners. It is known, however, that too much added sugar of all kinds — not just high-fructose corn syrup — can contribute unwanted calories that are linked to health problems, such as weight gain, type 2 diabetes, metabolic syndrome and high triglyceride levels. All of these boost your risk of heart disease.
Lipids
Lipids are a class of substances that are insoluble in water. They are hydrophobic (water-fearing).
There are three major groups of lipids:
1) Triglycerides - fats, oils, and waxes.
2) Phospholipids – makes up cell membranes. Will spontaneously form a membrane in water in the lab!
3) Steroids - hormones
Triglycerides consist of three fatty acids bonded to a glycerol molecule. Fatty acids are hydrocarbons (chains of covalently bonded carbons and hydrogens) with a carboxyl group (–COOH) at one end of the chain. A saturated fatty acid the maximum possible number of hydrogens bonded to it. The carbon back bone contains only single bonds. Unsaturated fatty acids have less hydrogen bonded to it due to having one or more double bonds. This double bond is a the CIS configuration. “Transfats” are unsaturated fats that have one or more double bonds in a TRANS configuration. [CIS (same) vs TRANS (opposite)] CIS fats are healthy fats that promote good cholesterol. TRANS fats, on the other hand, can be harmful and can contribute to a decrease in cardiovascular health. Examples of TRANS fats include substances that are made from unnatural sources like hydrogenated oils. In November 2013, the U.S. Food and Drug Administration (FDA) put forth a mandate that food companies eliminate TRANS fats from their products over time.
Phospholipids are the major component of cell membranes. Phospholipids are structurally similar to fats, except that they contain only two fatty acids attached to glycerol instead of three. they consist of a hydrophillic (polar) head and 2 hydrophobic (non-polar) tails.
Amphiphilic.
Phospholipids
Phospholipids spontaneously form a double-lipid bilayer (like the cell membrane) in water.
Chemistry may have brought forth “life”.
Steroids
PROTEINS
Nearly every dynamic function in your body depends on proteins.
A protein is a polymer of small building blocks called amino acids.
Of all of life’s molecules, proteins are structurally and functionally the most elaborate and varied.
Proteins
Proteins are polymers of amino acids.
Proteins perform a vast array of functions within organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another.
Making Polymers - dehydration reaction
One monomer loses a hydroxyl group (OH-) and the other monomer loses a hydrogen (H) atom to form H2O.
As this occurs, a new covalent bond forms, linking the two monomers.
Breaking Polymers
HYDROLYSIS
“hydro” = water
“lysis” = to cut
Cells not only make macromolecules but also have to break them down.
Most of the organic molecules in your food are in the form of polymers that are much too large to enter your cells.
Nucleic Acids
DNA and RNA are built from single units (monomers) of nucleic acids.
DNA = Deoxyribonucleic acid
RNA = Ribonucleic acid