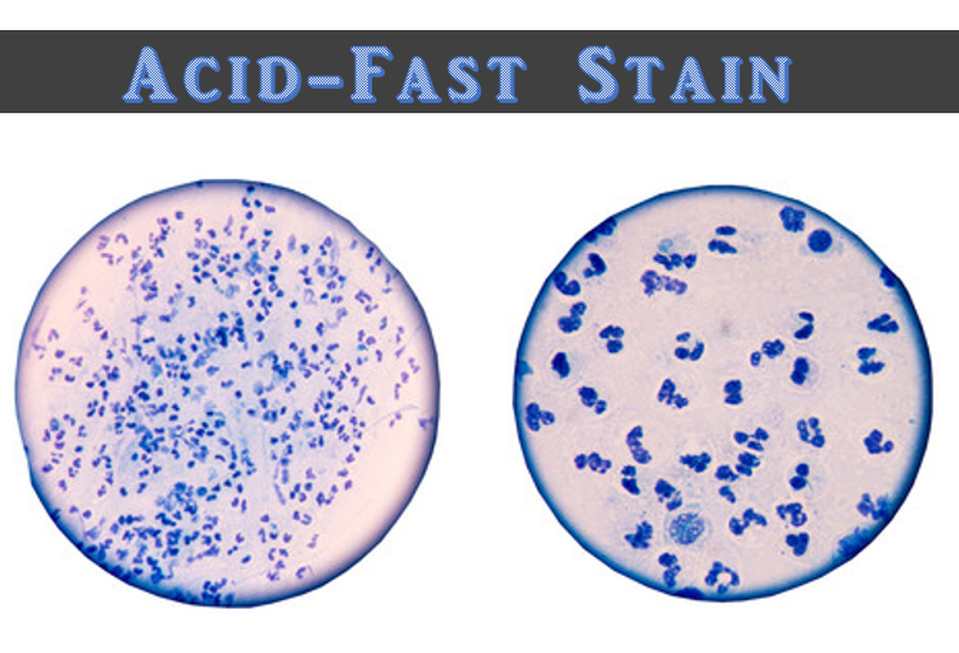
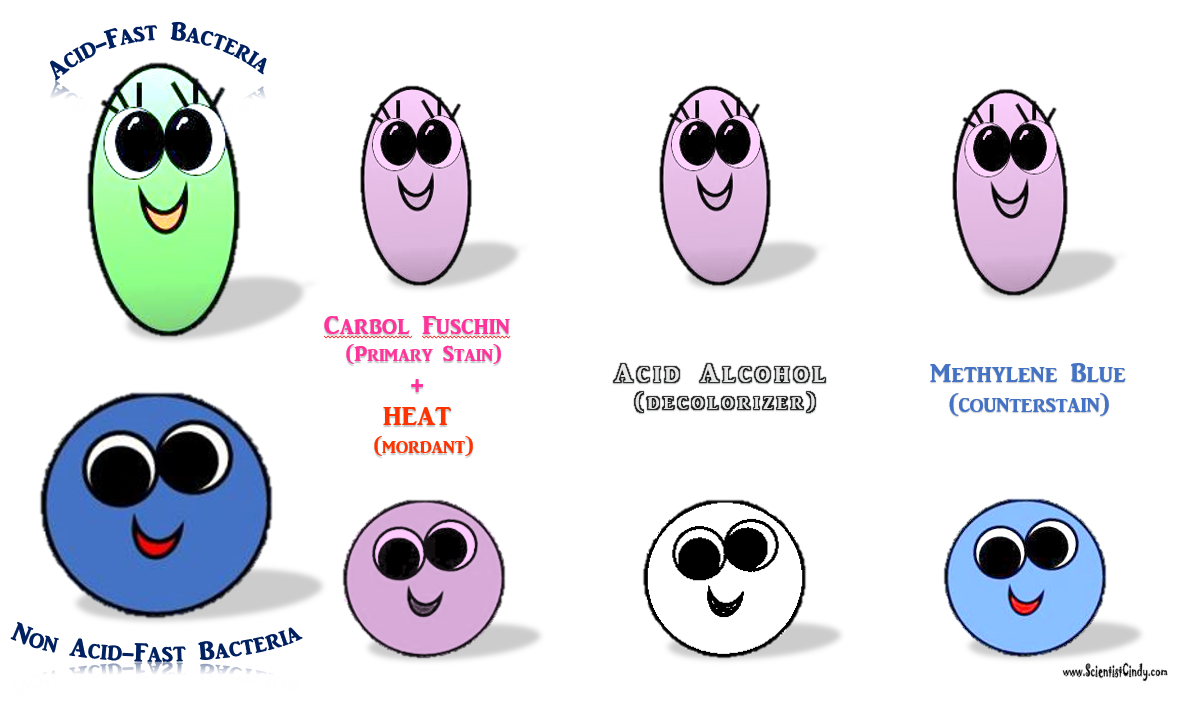
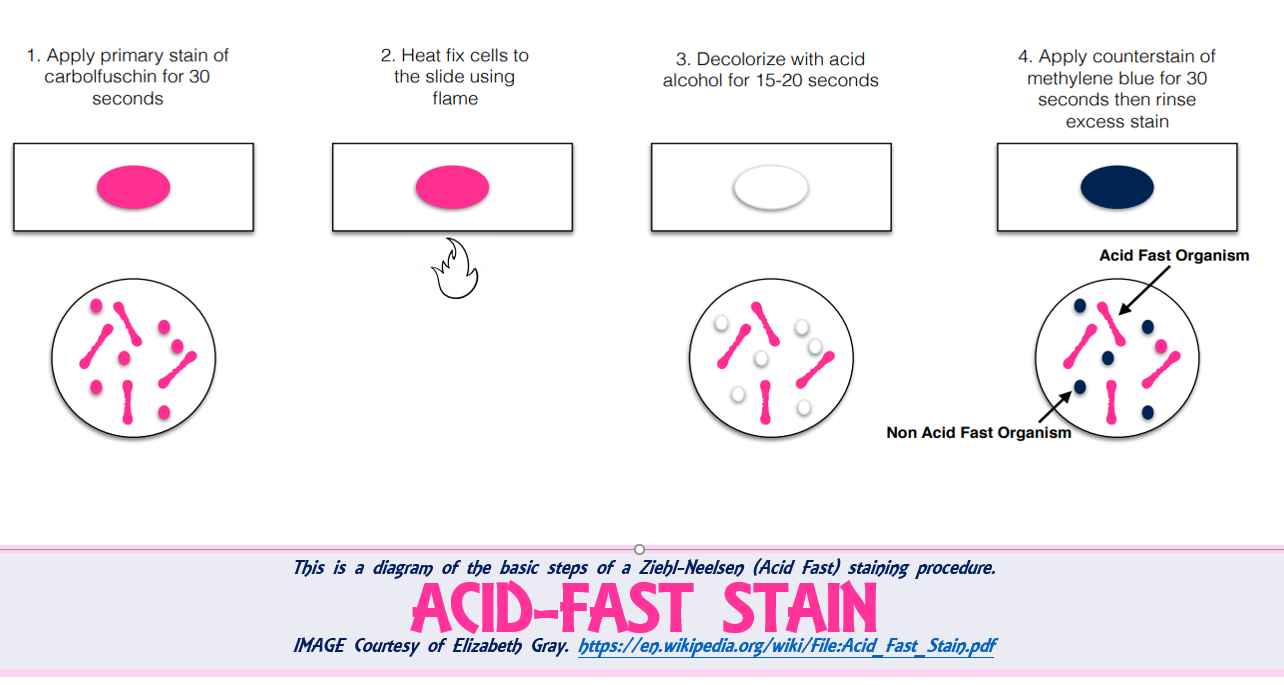
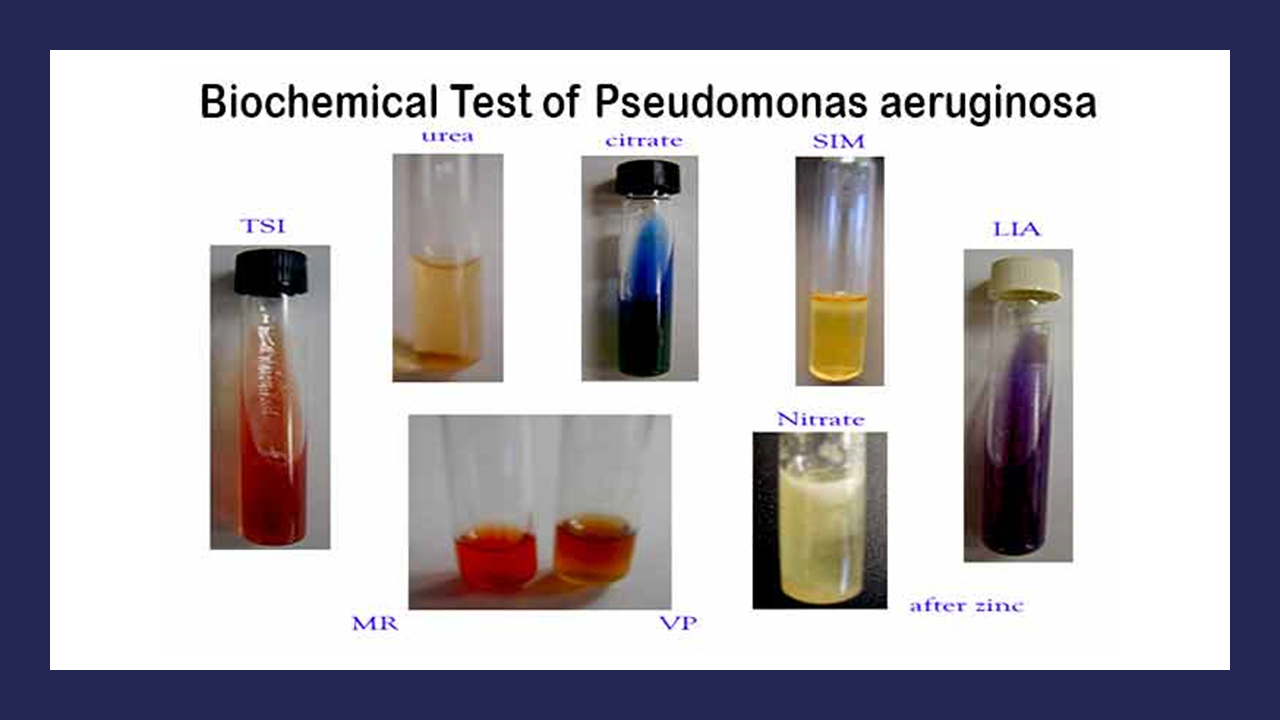
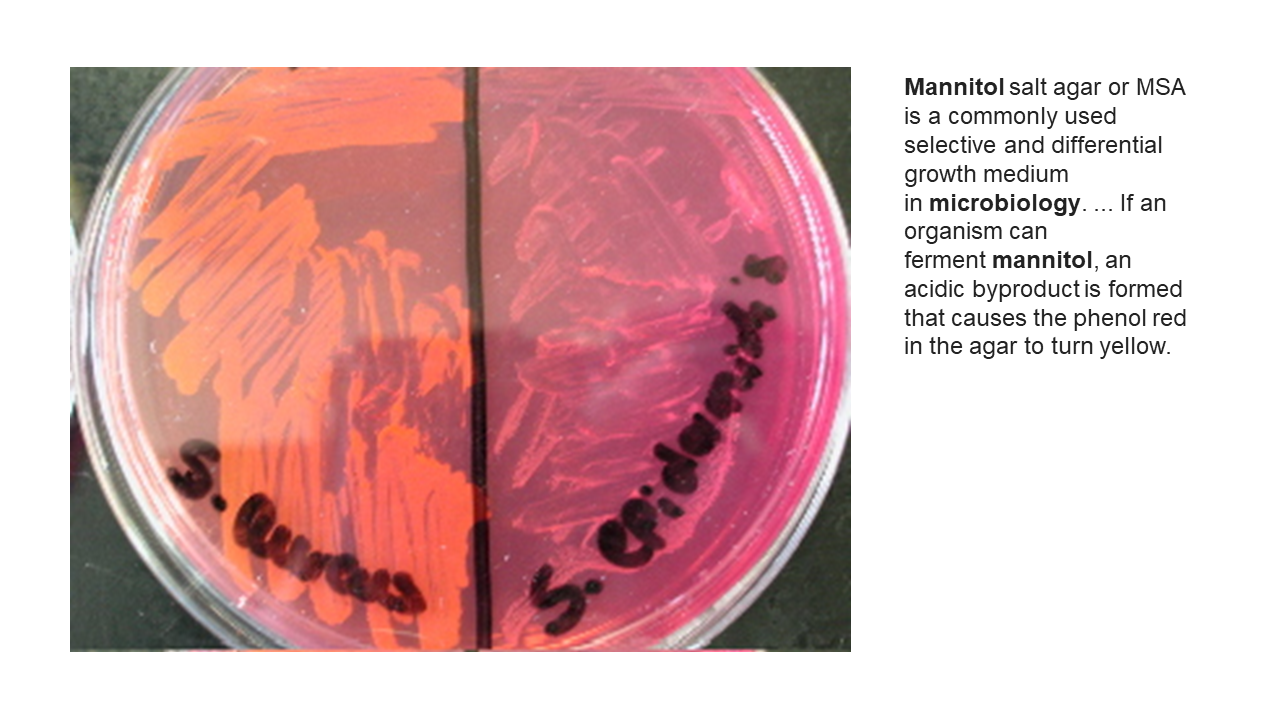
MICROBIOLOGY UNKNOWN LABORATORY
INOCULATIONS FOR NEXT
LAB PERIOD
1) Inoculate 2 Different TSA (Agar) Slants Using Single Line to Get a Growth Pattern
2) Inoculate 3 Different TSB (Broth) Tubes Using 3 Loop-Fulls of Your Unknown, to Determine the Growth Pattern in Broth and to Determine the Optimal Growth Temperature.

You will incubate the broth at different temperatures to determine the optimum growth temperature. 25 degrees Celsius (room temperature), 30 degrees Celsius, and 37 degrees Celsius are the temperatures that are best to test. When you are done with the incubations, you may be able to visualize which of the tubes had the most growth, by observing the cloudiness or (turbidity) of the sample. You can confirm your results, by measuring the % transmission.
•Determine optimum temperature for growth.
•Grow future cultures at this temperature.
•Determine optimum temperature for growth.
•Grow future cultures at this temperature.
3) 3 Thioglycolate to Determine Oxygen-Dependence
- •Obligate aerobes will only grow in the oxygen-rich top layer.
- •Obligate anaerobes will only grow in the lower areas of the tube.
- •Microaerophiles will grow in a thin layer below the richly-oxygenated layer.
- •Facultative or aerotolerant anaerobes can grow throughout the medium but will primarily grow in the middle of the tube, between the oxygen-rich and oxygen-free zones.
 Autoclave
Autoclave
•Oxygen is removed from the broth through autoclaving.
•Then, the broth is allowed to sit in unprotected in the presence of oxygen at room temperature, to allow oxygen to begins diffusing back into the broth.
•This is evidenced by the small layer of blue-green at the top of the broth.
•Then, the broth is allowed to sit in unprotected in the presence of oxygen at room temperature, to allow oxygen to begins diffusing back into the broth.
•This is evidenced by the small layer of blue-green at the top of the broth.

•Consistency/Texture :
- dry, moist, viscous (sticky), hard to get off, brittle (dry, breaks apart), mucoid (mucus-like)
- Some bacteria produce pigment when they grow in the medium e.g.,
- green pigment produces by Pseudomonas aeruginosa,
- buff colored colonies of Mycobacterium tuberculosis in L.J medium,
- red colored colonies of Serratia marcescens.
- Is the colony transparent (clear),
- opaque (not transparent or clear),
- translucent (almost clear, but distorted vision–like looking through frosted glass),
- iridescent (changing colors in reflected light).
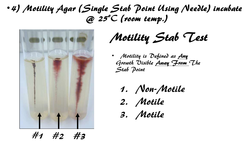
• For this test, a softer version of agar, called motility agar, will be used. Motility agar has a colorless dye (TTC or 2,3,5-Triphenyltetrazolium Chloride) that some bacteria have the ability to reduce. TTC becomes red when it is reduced by these bacteria.
• Bacteria that have the ability to reduce TTC, will reduce whatever TTC they may come into contact with. In this way, we will be able to visualize if the bacteria is motile or not, and if it is able to reduce TTC.
• Bacteria that have the ability to reduce TTC, will reduce whatever TTC they may come into contact with. In this way, we will be able to visualize if the bacteria is motile or not, and if it is able to reduce TTC.
MOTILITY STAB TEST
For this test, a softer version of agar, called motility agar, will be used. Motility agar has a colorless dye (TTC or 2,3,5-Triphenyltetrazolium Chloride) that some bacteria have the ability to reduce. TTC becomes red when it is reduced by these bacteria. You may want to review your oxidation-reduction reactions (a.k.a. red-ox reactions from general chemistry). Bacteria that have the ability to reduce TTC, will reduce whatever TTC they may come into contact with. In this way, we will be able to visualize if the bacteria is motile or not, and if it is able to reduce TTC. PROCEDURE FOR MOTILITY STAB TESTYou will prepare 2 separate samples (1 using S. aureus and 1 using P. vulgaris) in exactly the same way, using sterile technique.
For this test, a softer version of agar, called motility agar, will be used. Motility agar has a colorless dye (TTC or 2,3,5-Triphenyltetrazolium Chloride) that some bacteria have the ability to reduce. TTC becomes red when it is reduced by these bacteria. You may want to review your oxidation-reduction reactions (a.k.a. red-ox reactions from general chemistry). Bacteria that have the ability to reduce TTC, will reduce whatever TTC they may come into contact with. In this way, we will be able to visualize if the bacteria is motile or not, and if it is able to reduce TTC. PROCEDURE FOR MOTILITY STAB TESTYou will prepare 2 separate samples (1 using S. aureus and 1 using P. vulgaris) in exactly the same way, using sterile technique.
- Flame inoculation needle, uncap agar tube of appropriate bacteria and stab 2-3 times in same spot.
- Flame the mouth of the tube and recap tube., then remove cap from the motility agar and stab the motility agar ONE TIME ONLY. Flame mouth of motility agar tube and replace cap. Flame inoculation needle well (until visibly red) to sterilize.
- Incubate this media at 25°C.
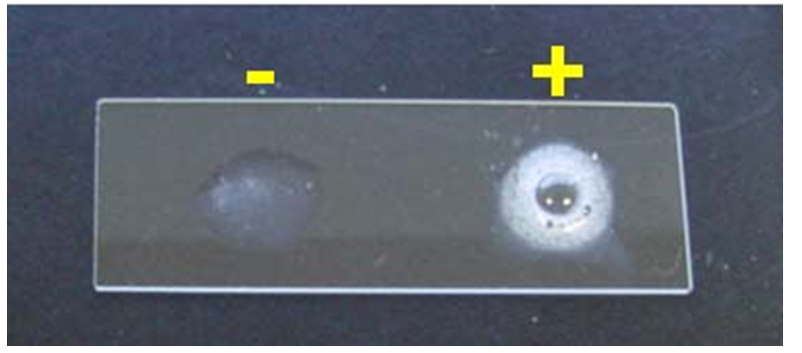
Microorganisms able to live in oxygenated environments produce enzymes which neutralize toxic forms of oxygen. One such enzyme is catalase, which breaks hydrogen peroxide into water and molecular oxygen. Organisms which produce catalase will bubble when placed into hydrogen peroxide. The catalase test is primarily used to distinguish among Gram-positive cocci: Member of the genus Staphylococcus are catalase-positive, and members of the genera Streptococcus and Enterococcus are catalase-negative.
Gram-Negative Bacteria Dichotomous Chart
Gram-Positive Bacteria Dichotomous Chart
Fermentation Tests
Bubble = positive
No bubble = negative
Bubble = positive
No bubble = negative

Indole Test
Some bacteria are able to produce indole. Bacteria use an enzyme, tryptophanase to break down the amino acid, tryptophan, which makes by-products, including indole .
Media and Reagents Used: Tryptone broth contains tryptophan. Kovac’s reagent—contains hydrochloric acid, dimethylaminobenzaldehyde, and amyl alcohol—yellow in color. Reading Results: Kovac’s reagent reacts with indole and creates a red color at the top part of the test tube.
Some bacteria are able to produce indole. Bacteria use an enzyme, tryptophanase to break down the amino acid, tryptophan, which makes by-products, including indole .
Media and Reagents Used: Tryptone broth contains tryptophan. Kovac’s reagent—contains hydrochloric acid, dimethylaminobenzaldehyde, and amyl alcohol—yellow in color. Reading Results: Kovac’s reagent reacts with indole and creates a red color at the top part of the test tube.
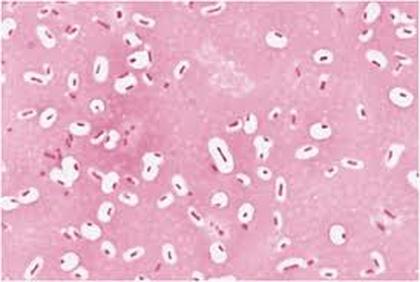
CAPSULE STAIN -
Capsule stain involves a negative stain that colors the background and a dye that stains the bacterial cells, so that the capsule can be seen. The capsule surrounds the outermost portion of the cell and functions as added protection.
Capsule stain involves a negative stain that colors the background and a dye that stains the bacterial cells, so that the capsule can be seen. The capsule surrounds the outermost portion of the cell and functions as added protection.
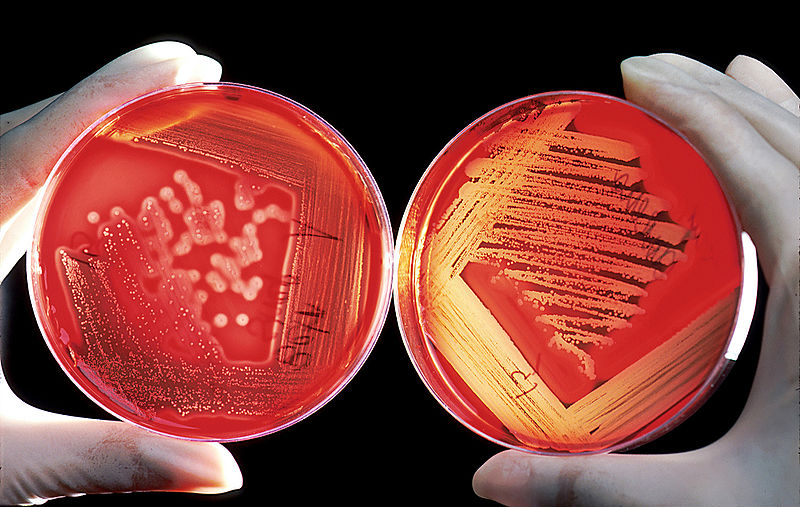
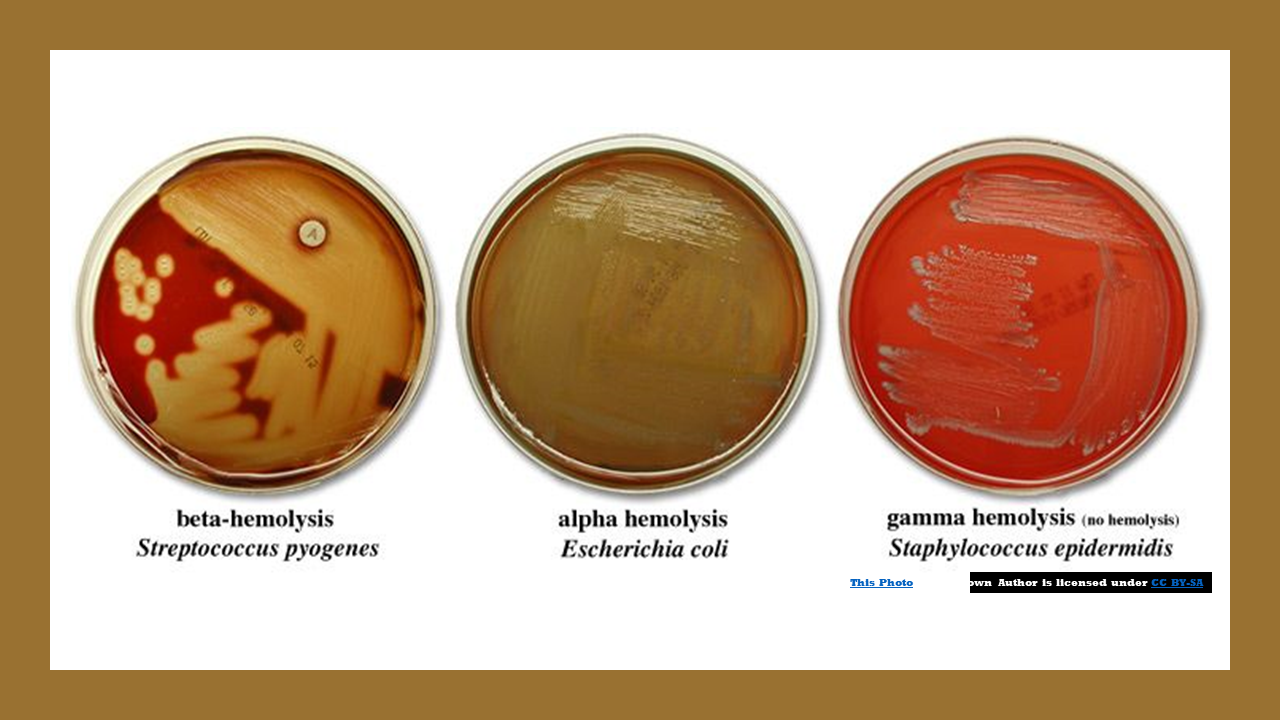
Blood agar is a bacterial growth medium used to grow fastidious organisms that need a lot of nutrients. Blood agar is composed of tryptic soy agar enriched with 5% sheep blood.
Blood Agar and Hemolysis
Some bacterial secrete enzymes (exoenzymes) that lyse red blood cells in the blood agar (hemolysis).
Some bacterial secrete enzymes (exoenzymes) that lyse red blood cells in the blood agar (hemolysis).
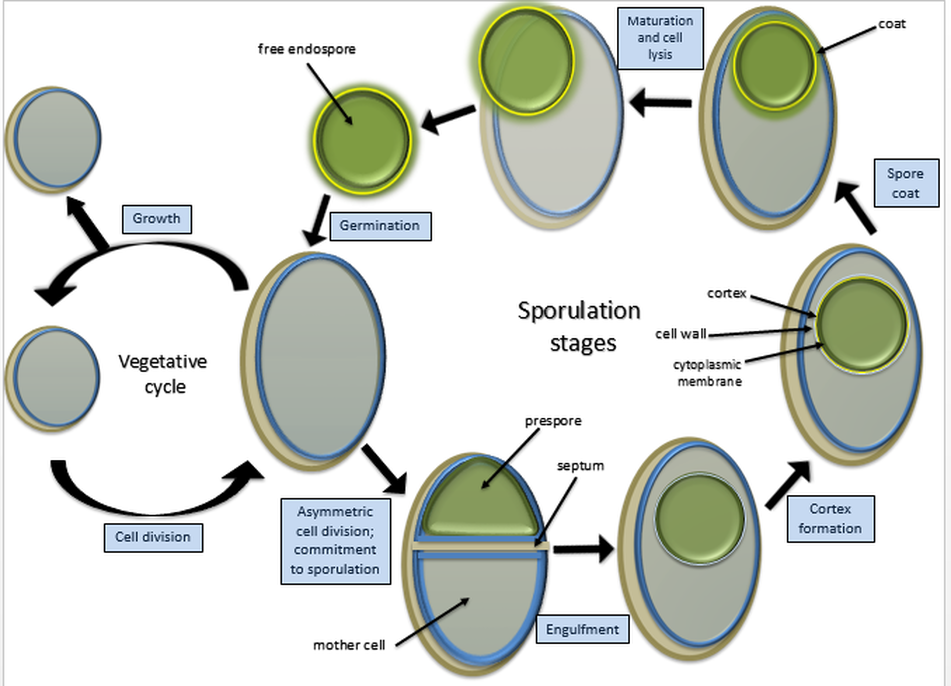
Some Gram-positive bacteria, are able to survive unfavorable environmental conditions for extended periods of time (can be hundreds of years) by forming protective endospores through a process called sporulation
Bacteria that have endospores are existing in a semi-dormant state of existence. The endospores preserves the genetic material of the bacteria cell forms of bacteria. When environmental conditions improve, the endospore can undergo the germination process and can return to its normal (vegetative) state.
Endospores protect the bacterial DNA from dehydration, pH, heat and radiation. 2 examples of Gram-positive bacteria that have the ability to undergo sporulation are:
Some species of Bacillus and Clostridium have medical significance. Clostridium perfringens, C. botulinum (a potential agent of bioterrorism) and C. tetani are the causative agents of gas gangrene, botulism and tetanus, respectively. Bacillus anthracisand Bacillus cereus are the causative agents of anthrax and a self limiting food poisoning, respectively.
Endospores protect the bacterial DNA from dehydration, pH, heat and radiation. 2 examples of Gram-positive bacteria that have the ability to undergo sporulation are:
- Bacillus
- Clostridia
Some species of Bacillus and Clostridium have medical significance. Clostridium perfringens, C. botulinum (a potential agent of bioterrorism) and C. tetani are the causative agents of gas gangrene, botulism and tetanus, respectively. Bacillus anthracisand Bacillus cereus are the causative agents of anthrax and a self limiting food poisoning, respectively.

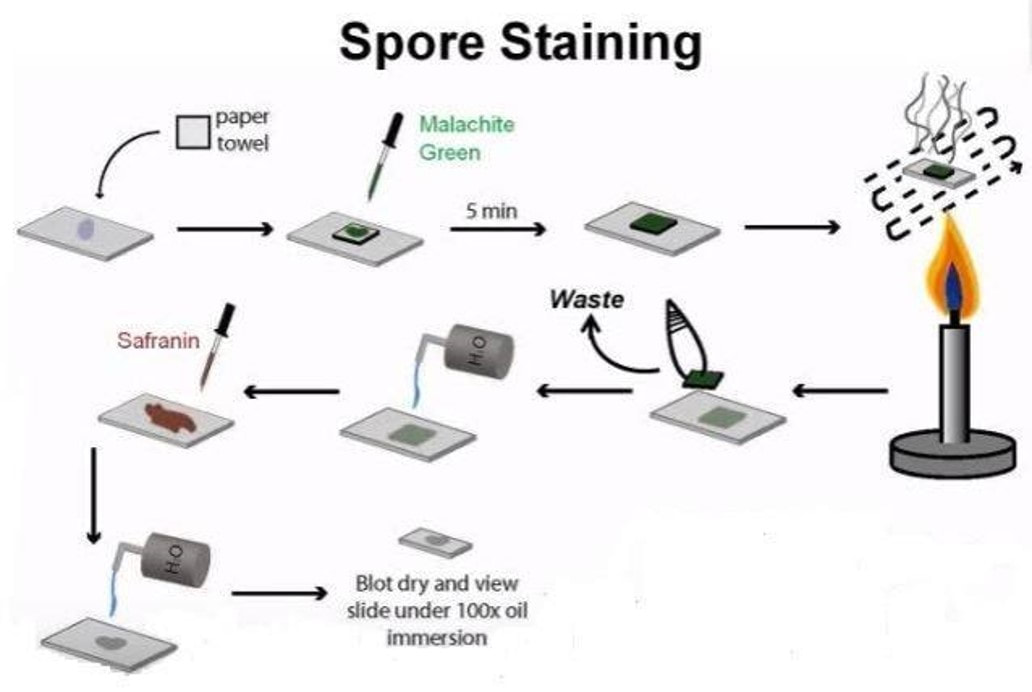
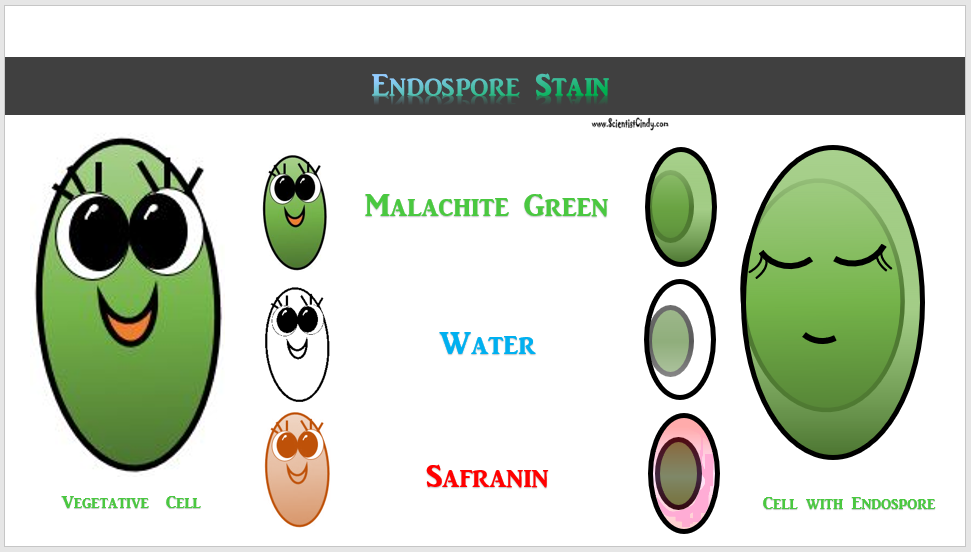
Endospores are also not easily penetrated by staining procedures. Heat needs to be applied to help the stain enter the endospore. This heating process acts as the mordant. In this staining procedure, the endospore will be green due to the malachite green and the vegetative cell will be red due to the safranin. The endospore stain is a differential stain used to visualize bacterial endospores. Endospores are formed by a few genera of bacteria, such as Bacillus . By forming spores, bacteria can survive in hostile conditions. Spores are resistant to heat, dessication, chemicals, and radiation.
Endospore Staining is helpful for identifying bacteria that have endospores. Endospores are protective structures that are used to withstand unfavorable conditions. Endospores are difficult to stain using normal techniques like Gram-stain and simple stains.
DETAILS OF THE STAINING PROCEDURE
The primary dye for endospore staining is malachite green.
It takes a long time for the spores to stain due to their density, so time acts as the mordant when performing this differential stain; the slide with the bacterium should be soaked in malachite green for at least 30 minutes and then rinsed off with water which acts as the decolorizer.
A counterstain to differentiate the vegetative cells is commonly 0.5% safranin.
RESULTS
The endospores stain green and the rest of the cells picks up the pinkish-red counterstain. The endospore will often appear as a green spot within the cell.
DETAILS OF THE STAINING PROCEDURE
The primary dye for endospore staining is malachite green.
It takes a long time for the spores to stain due to their density, so time acts as the mordant when performing this differential stain; the slide with the bacterium should be soaked in malachite green for at least 30 minutes and then rinsed off with water which acts as the decolorizer.
A counterstain to differentiate the vegetative cells is commonly 0.5% safranin.
RESULTS
The endospores stain green and the rest of the cells picks up the pinkish-red counterstain. The endospore will often appear as a green spot within the cell.
The carbohydrate fermentation test is used to determine whether or not bacteria can ferment a specific carbohydrate. Carbohydrate fermentation patterns are useful in differentiating among bacterial groups or species.
It tests for the presence of acid and/or gas produced from carbohydrate fermentation.
Broth with a single carbohydrate source such as Glucose, Lactose, Sucrose, etc. along with a pH indicator.
It tests for the presence of acid and/or gas produced from carbohydrate fermentation.
Broth with a single carbohydrate source such as Glucose, Lactose, Sucrose, etc. along with a pH indicator.