INTRODUCTION TO THE CIRCULATORY SYSTEM The Components of the Circulatory System
Before we begin, let's start with some basic concepts and definitions. Let's first discuss WHAT the circulatory system IS and WHAT the circulatory system DOES for us.
The function of the circulatory system is to transport the blood throughout the body.
The circulatory system has 3 components:
The function of the circulatory system is to transport the blood throughout the body.
The circulatory system has 3 components:
- The heart - functions to pump blood throughout the body by creating a pressure gradient.
- The blood vessels - the network of passage ways for the blood to travel through.
- The blood - carries the substances that are being transported to and from the cells.
Components of the BloodThe blood has 3 major components:
|
|
THE RED BLOOD CELLS (ERYTHROCYTES)
The proper name for Red Blood Cells is Erythrocytes. Red blood cells are short-lived and do not have a nucleus. The main function of the erythrocytes is to
The proper name for Red Blood Cells is Erythrocytes. Red blood cells are short-lived and do not have a nucleus. The main function of the erythrocytes is to
- transport oxygen to the cells of the body
- take away carbon dioxide from the cells of the body
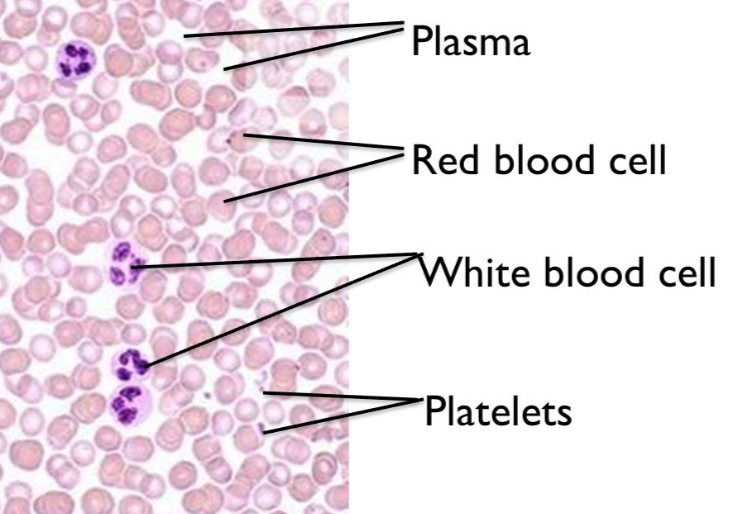
The red blood cells are rather easy to spot on slides, because they do not have a nucleus!
White blood cells do have a nucleus which stains purple with hematoxylin.
Platelets appear as small particles.
Blood plasma is the liquid substance that the cells and platelets reside in inside of the blood vessels.
White blood cells do have a nucleus which stains purple with hematoxylin.
Platelets appear as small particles.
Blood plasma is the liquid substance that the cells and platelets reside in inside of the blood vessels.
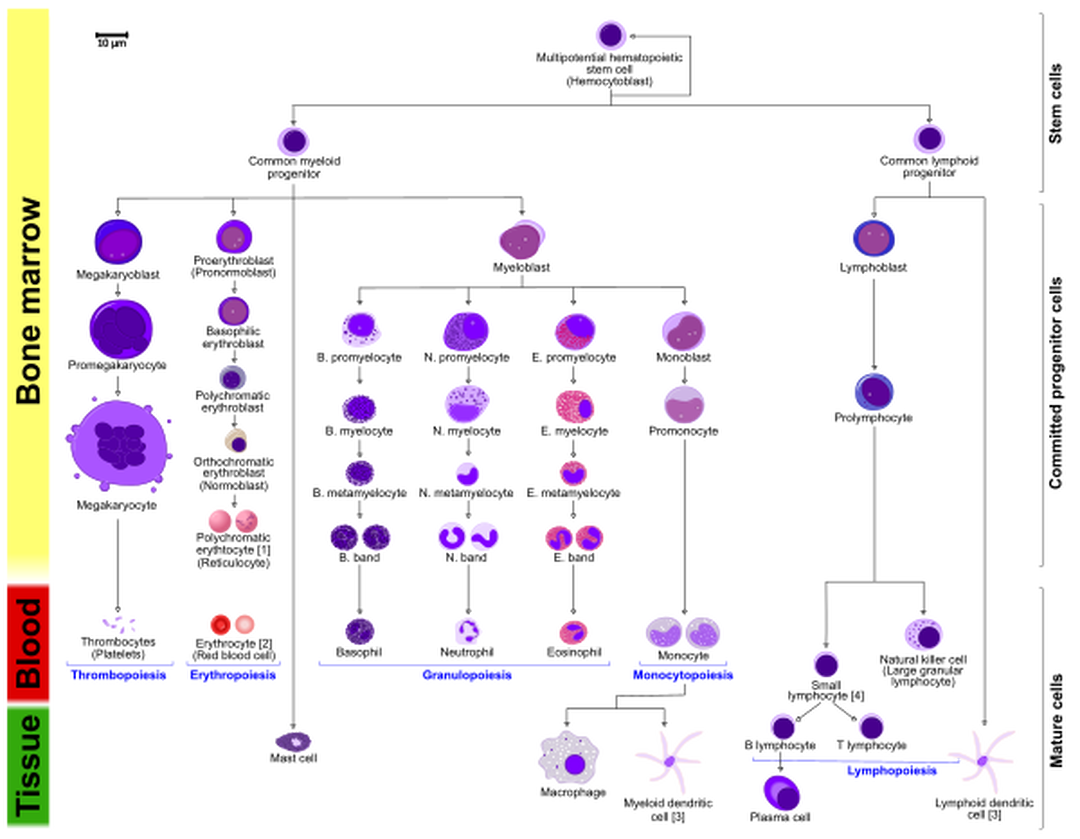
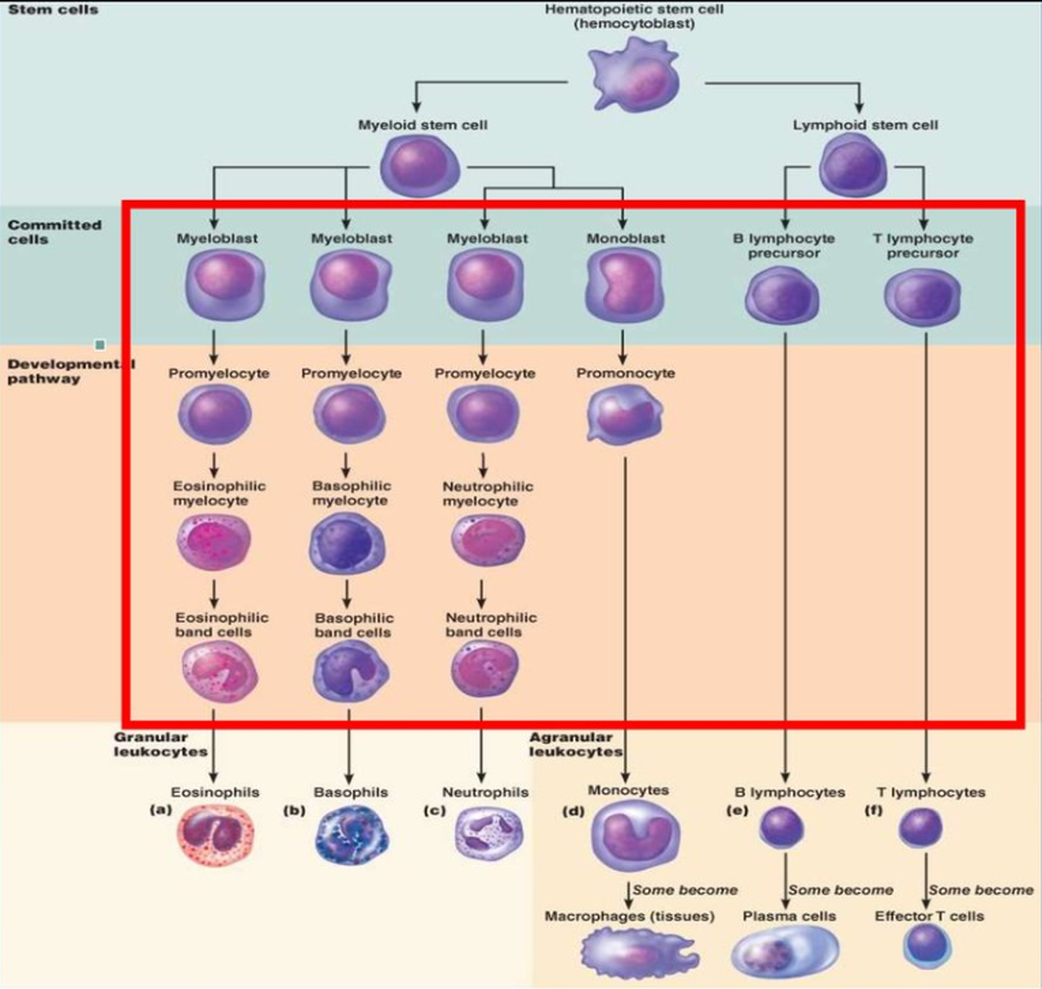
Haematopoiesis is the formation of blood components. All cellular blood components come from haematopoietic stem cells. Haematopoietic stem cells are located in bone marrow. These stem cells have the ability to give rise to all of the different blood cell types and components. All blood cells are divided into three lineages.
- Red blood cells, also called erythrocytes, are the oxygen-carrying cells.
- Lymphocytes are the cornerstone of the adaptive immune system. They are derived from common lymphoid progenitors. The lymphoid lineage is composed of T-cells, B-cells and natural killer cells.
- Cells of the myeloid lineage, which include granulocytes, megakaryocytes and macrophages, are derived from common myeloid progenitors, and are involved in such diverse roles as innate immunity and blood clotting.
Overview of Blood
The primary function of blood is to supply oxygen and nutrients to the cells of the body and to remove their waste products.
Problems with blood composition or circulation can lead to illness and disease.
The blood has an especially important roll in maintaining homeostasis by acting as a medium for transferring heat to the skin and by acting as a buffer system for bodily pH.
The primary function of blood is to supply oxygen and nutrients to the cells of the body and to remove their waste products.
Problems with blood composition or circulation can lead to illness and disease.
The blood has an especially important roll in maintaining homeostasis by acting as a medium for transferring heat to the skin and by acting as a buffer system for bodily pH.
The blood is circulated through the lungs and body by the pumping action of the heart. The right ventricle pressurizes the blood to send it through the capillaries of the lungs, while the left ventricle re-pressurizes the blood to send it throughout the body. Pressure is essentially lost in the capillaries, hence gravity and especially the actions of skeletal muscles are needed to return the blood to the heart.
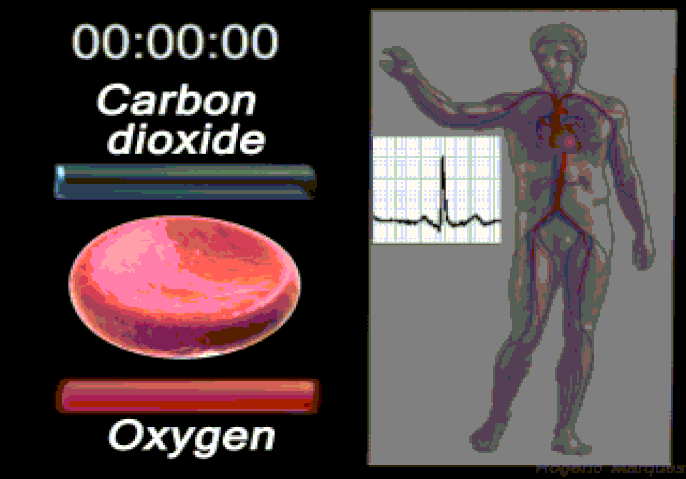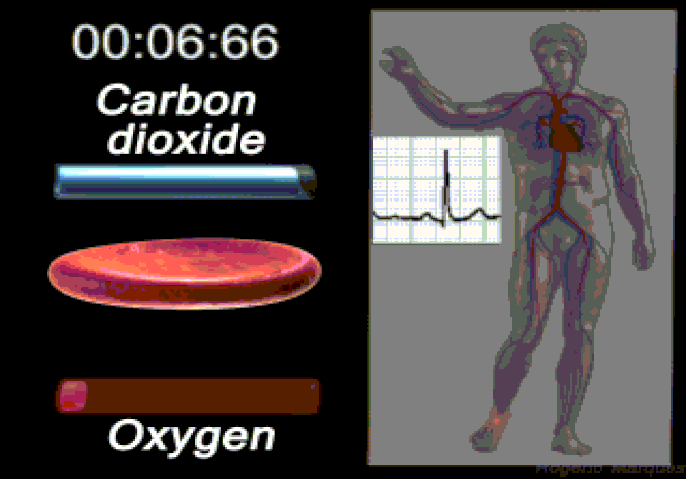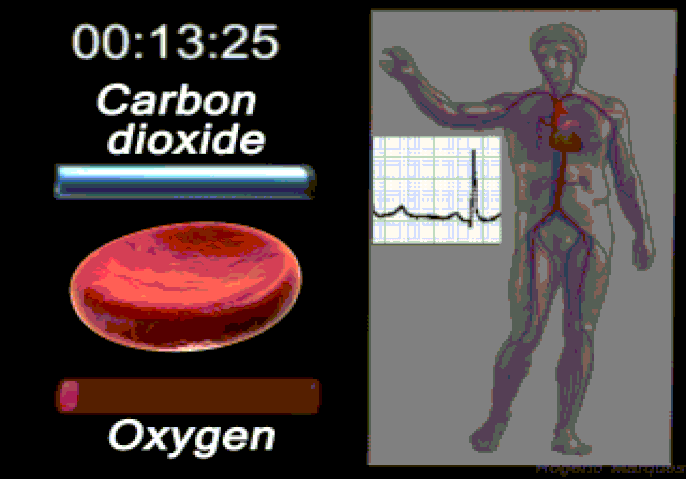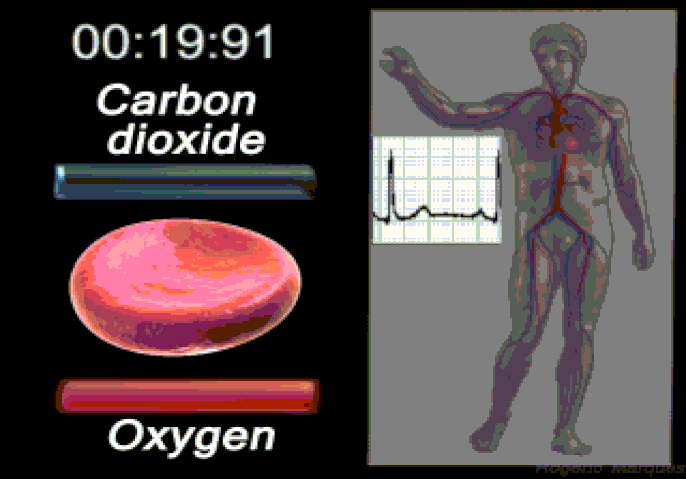
Oxygen (O2) is the most immediate need of every cell and is carried throughout the body by the blood circulation. Oxygen is used at the cellular level as the final electron acceptor in the electron transport chain (the primary method of generating ATP for cellular reactions). Oxygen is carried in the blood bound to hemoglobin molecules within red blood cells. Hemoglobin binds oxygen when passing through the alveoli of the lungs and releases oxygen in the warmer, more acidic environment of bodily tissues, via simple diffusion.
Carbon dioxide (CO2) is removed from tissues by blood and released into the air via the lungs.
Carbon dioxide is produced by cells as they undergo the processes of cellular respiration (particularly the Kreb's Cycle). The molecules are produced from carbons that were originally part of glucose. Most of the carbon dioxide combines with water and is carried in the plasma as bicarbonate ions.
An excess of carbon dioxide (through exercise, or from holding ones breath) quickly shifts the blood pH to being more acidic (acidosis). Chemoreceptors in the brain and major blood vessels detect this shift and stimulate the breathing center of the brain (the medulla oblongata). Hence, as CO2 levels build up and the blood becomes more acidic, we involuntarily breathe faster, thus lowering CO2 levels and stabilizing blood pH. In contrast, a person who is hyperventilating (such as during a panic attack) will expire more CO2 than being produced in the body and the blood will become too alkaline (alkalosis).
Oxygen (O2) is the most immediate need of every cell and is carried throughout the body by the blood circulation. Oxygen is used at the cellular level as the final electron acceptor in the electron transport chain (the primary method of generating ATP for cellular reactions). Oxygen is carried in the blood bound to hemoglobin molecules within red blood cells. Hemoglobin binds oxygen when passing through the alveoli of the lungs and releases oxygen in the warmer, more acidic environment of bodily tissues, via simple diffusion.
Carbon dioxide (CO2) is removed from tissues by blood and released into the air via the lungs. Carbon dioxide is produced by cells as they undergo the processes of cellular respiration (particularly the Kreb's Cycle). The molecules are produced from carbons that were originally part of glucose. Most of the carbon dioxide combines with water and is carried in the plasma as bicarbonate ions. An excess of carbon dioxide (through exercise, or from holding ones breath) quickly shifts the blood pH to being more acidic (acidosis). Chemoreceptors in the brain and major blood vessels detect this shift and stimulate the breathing center of the brain (the medulla oblongata). Hence, as CO2 levels build up and the blood becomes more acidic, we involuntarily breathe faster, thus lowering CO2 levels and stabilizing blood pH. In contrast, a person who is hyperventilating (such as during a panic attack) will expire more CO2 than being produced in the body and the blood will become too alkaline (alkalosis).
Carbon dioxide (CO2) is removed from tissues by blood and released into the air via the lungs. Carbon dioxide is produced by cells as they undergo the processes of cellular respiration (particularly the Kreb's Cycle). The molecules are produced from carbons that were originally part of glucose. Most of the carbon dioxide combines with water and is carried in the plasma as bicarbonate ions. An excess of carbon dioxide (through exercise, or from holding ones breath) quickly shifts the blood pH to being more acidic (acidosis). Chemoreceptors in the brain and major blood vessels detect this shift and stimulate the breathing center of the brain (the medulla oblongata). Hence, as CO2 levels build up and the blood becomes more acidic, we involuntarily breathe faster, thus lowering CO2 levels and stabilizing blood pH. In contrast, a person who is hyperventilating (such as during a panic attack) will expire more CO2 than being produced in the body and the blood will become too alkaline (alkalosis).
Blood is made of two parts:
- Plasma which makes up 55% of blood volume.
- Formed cellular elements (red and white blood cells, and platelets) which combine to make the remaining 45% of blood volume.
Plasma makeup
Plasma is made up of 90% water, 7-8% soluble proteins, 1% carbon-dioxide and oxygen, and 1% is salt, which helps with the pH of the blood. Plasma also carries Respiratory gases; CO2 in large amounts(about 97%) and O2 in small amounts(about 3%), various nutrients(glucose, fats), wastes of metabolic exchange(urea, ammonia), hormones, and vitamins. The largest group of solutes in plasma contains three important proteins like albumins, globulins, and clotting proteins.
Plasma is made up of 90% water, 7-8% soluble proteins, 1% carbon-dioxide and oxygen, and 1% is salt, which helps with the pH of the blood. Plasma also carries Respiratory gases; CO2 in large amounts(about 97%) and O2 in small amounts(about 3%), various nutrients(glucose, fats), wastes of metabolic exchange(urea, ammonia), hormones, and vitamins. The largest group of solutes in plasma contains three important proteins like albumins, globulins, and clotting proteins.
- The main function of albumins is to maintain the osmotic balance between the blood and tissue fluids and is called colloid osmotic pressure. In addition, albumins assist in transport of different materials, such as vitamins and certain molecules and drugs (e.g. bilirubin, fatty acids, and penicillin).
- Globulins are a diverse group of proteins, designated into three groups: gamma, alpha, and beta. Their main function is to transport various substances in the blood. Gamma globulins assist the body's immune system in defense against infections and illness.
- Clotting proteins are mainly produced in the liver as well. There are at least 12 substances, known as "clotting factors" that participate in the clotting process.
Red blood cell (erythrocyte) also known as "RBCs". RBCs are formed in the red bone marrow. The formation of RBCs is called erythropoiesis. Red blood cells lose nuclei upon maturation, and take on a biconcave, dimpled, shape. They are about 7-8 micrometers in diameter. There are about 1000x more red blood cells than white blood cells. RBCs live about 120 days and do not self repair. RBCs contain hemoglobin which transports oxygen from the lungs to the rest of the body, such as to the muscles, where it releases the oxygen load.The hemoglobin gets its red color from their respiratory pigments.
Shape
RBCS have a shape of a disk that appears to be “caved in” or almost flattened in the middle; this is called bi-concave. This bi-concave shape allows the RBC to carry oxygen and pass through even the smallest capillaries in the lungs. This shape also allows RBCs to stack like dinner plates and bend as they flow smoothly through the narrow blood vessels in the body. RBCs lack a nucleus (no DNA) and no organelles, meaning that these cells cannot divide or replicate themselves like the cells in our skin and muscles. RBCs have a short life span of about 120 days, however, as long as our myeloid tissue is working correctly, we will produce about 2-3 million RBCs per second. That is about 200 billion a day! This allows us to have more to replace the ones we lose.
Hemaglobin
The main component of the RBC is hemoglobin. Hemoglobin is protein that is composed of four protein subunits. Hemoglobin is responsible for the cell’s ability to transport oxygen and carbon dioxide. Hemoglobin, iron, and oxygen interact with each other, forming the RBCs' bright red color. You can call this interaction by product oxyhemoglobin. Carbon Monoxide binds with hemoglobin faster than oxygen, and stays bound for several hours, making hemoglobin temporarily unavailable for oxygen transport. One red blood cell contains about 200 million hemoglobin molecules. If all this hemoglobin was in the plasma rather than inside the cells, blood would be so "thick" that the heart would have a difficult time pumping it through. The thickness of blood is called viscosity. The greater the viscosity of blood, the more friction there is, and more pressure is needed to force blood through.
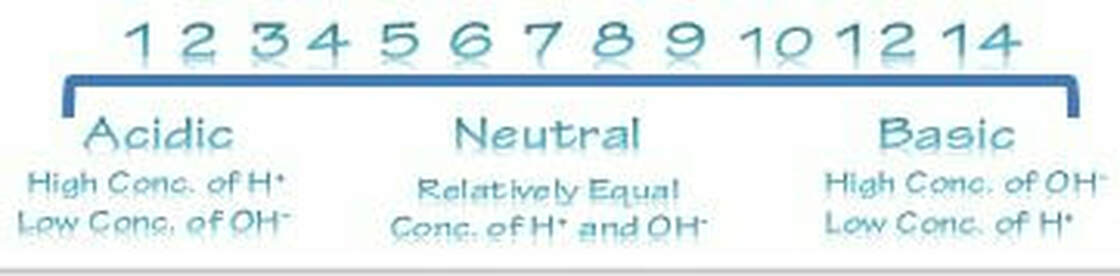
Functions[edit]The main function is the transportation of oxygen throughout the body and the ability of the blood to carry out carbon dioxide which is called carbamino – hemoglobin. Maintaining the balance of blood is important. The balance can be measured by the acid and base levels in the blood. This is called pH. Normal pH of blood ranges between 7.35-7.45; this normal blood is called Alkaline (less acidic then water). A drop in pH is called Acidic. This condition is also called Acidosis. A jump in pH higher then 7.45 is called "Alkalosis". To maintain the homeostasis (or balance,) the blood has tiny molecules within the RBC that help prevent drops or increases from happening.
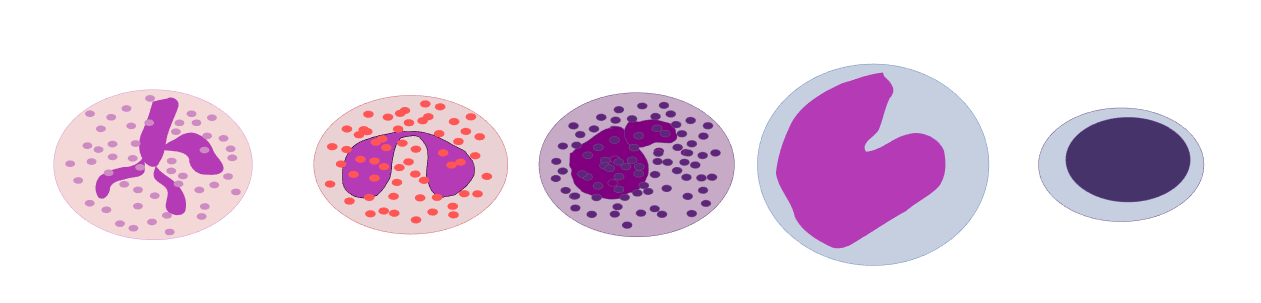
From left to right diagram of Erythrocyte, Thrombocyte, and LeukocyteDestruction
Red blood cells are broken down and hemoglobin is released. The globin part of the hemoglobin is broken down into amino acid components, which in turn are recycled by the body. The iron is recovered and returned to the bone marrow to be reused. The heme portion of the molecule experiences a chemical change and then gets excreted as bile pigment (bilirubin) by the liver. Heme portion after being broken down contributes to the color of feces and your skin color changing after being bruised.
White Blood Cells[edit]Shape
White blood cells are different from red cells in the fact that they are usually larger in size 10-14 micrometers in diameter. White blood cells do not contain hemoglobin which in turn makes them translucent. Many times in diagrams or pictures white blood cells are represented in a blue color, mainly because blue is the color of the stain used to see the cells. White blood cells also have nucleii, that are some what segmented and are surrounded by electrons inside the membrane.
Functions[edit]White blood cells (leukocytes) are also known as "WBC's". White blood cells are made in the bone marrow but they also divide in the blood and lymphatic systems. They are commonly amoeboid (cells that move or feed by means of temporary projections, called pseudopods (false feet), and escape the circulatory system through the capillary beds. The different types of WBC's are Basophils, Eosinophils, Neutrophils, Monocytes, B- and T-cell lymphocytes. Neutrophils, Eosinophils, and Basophils are all granular leukocytes. Lymphocytes and Monocytes are agranular leukocytes. Basophils store and synthesize histamine which is important in allergic reactions. They enter the tissues and become "mast cells" which help blood flow to injured tissues by the release of histamine. Eosinophils are chemotoxic and kill parasites. Neutrophils are the first to act when there is an infection and are also the most abundant white blood cells. Neutrophils fight bacteria and viruses by phagocytosis which mean they engulf pathogens that may cause infection. The life span of a of Neutrophil is only about 12-48 hours. Monocytes are the biggest of the white blood cells and are responsible for rallying the cells to defend the body. Monocytes carry out phagocytosis and are also called macrophages. Lymphocytes help with our immune response. There are two Lymphocytes: the B- and T- cell. B-Lymphocytes produce antibodies that find and mark pathogens for destruction. T-Lymphocytes kill anything that they deem abnormal to the body.
WBCs are classified by phenotype which can be identified by looking at the WBCs under a microscope. The Granular phenotype are able to stain blue. The Agranular phenotype are able to stain red. Neutrophils make up 50-70% of Granular cells Eosinophils make up 2-4%, and Basophils 0-1%. Monocytes make up 2-8% of Agranular cells. B and T Lymphocytes make up 20-30%. As you can see, there is a great deal of differentiation between WBCs. These special cells help our bodies defend themselves against pathogens. Not only do they help our immune system but they remove toxins, wastes, and abnormal or damaged cells. Thus, we can say that WBCs' main function is being Phagocytic which means to engulf or swallow cells.
Platelets[edit]
A 250 ml bag of newly collected platelets.Platelets, also called thrombocytes, are membrane-bound cell fragments. Platelets have no nucleus, they are between one to two micrometers in diameter, and are about 1/10th to 1/20th as abundant as white blood cells. Less than 1% of whole blood consists of platelets. They result from fragmentation of large cells called Megakaryocytes - which are cells derived from stem cells in the bone marrow. Platelets are produced at a rate of 200 billion per day. Their production is regulated by the hormone called Thrombopoietin. The circulating life of a platelet is 8–10 days. The sticky surface of the platelets allow them to accumulate at the site of broken blood vessels to form a clot. This aids in the process of hemostasis ("blood stopping"). Platelets secrete factors that increase local platelet aggregation (e.g., Thromboxane A), enhance vasoconstriction (e.g., Serotonin), and promote blood coagulation (e.g., Thromboplastin).
Hemostasis (Coagulation or Clotting)[edit]Hemostasis is the natural process of stopping blood flow or loss of blood following an injury. (hemo = blood; stasis = standing). It has three stages: (1) vascular spasm, vasoconstriction, or intense contraction of blood vessels, (2) formation of a platelet plug and (3) blood clotting or coagulation. Once the flow of blood has been stopped, tissue repair can begin.
Vascular spasm or Vasoconsriction: In a normal individual, immediately after a blood vessel has been cut and endothelial cells are damaged, vasoconstriction occurs, thus slowing blood flow to the area. Smooth muscle in the vessel wall goes through spasms or intense contractions that constrict the vessel. If the vessels are small, spasms compress the inner walls together and may be able to stop the bleeding completely. If the vessels are medium to large-sized, the spasms slow down immediate outflow of blood, lessening the damage but still preparing the vessel for the later steps of hemostasis. These vascular spasms usually last for about 30 minutes, long enough for the next two stages of hemostasis to take place.
Formation of a Platelet Plug: Within 20 seconds of an injury, coagulation is initiated. Contrary to popular belief, clotting of a cut on the skin is not initiated by air or drying out, but by platelets adhering to and activated by collagen in the blood vessels endothelium. The activated platelets then release the contents of their granules, which contain a variety of substances that stimulate further platelet activation and enhance the hemostatic process.
When the lining of a blood vessel breaks and endothelial cells are damaged, revealing collagen proteins in the vessel wall, platelets swell, grow spiky extensions, and start clumping together. They start to stick to each other and the walls of the vessel. This continues as more platelets congregate and undergo these same transformations. This process results in a platelet plug that seals the injured area. If the injury is small, a platelet plug may be able to form and close it within several seconds. If the damage is more serious, the next step of blood clotting will take place. Platelets contain secretory granules. When they stick to the proteins in the vessel walls, they degranulate, thus releasing their products, which include ADP (adenosine diphosphate), serotonin, and thromboxane A2.
A Blood Clot Forms: If the platelet plug is not enough to stop the bleeding, the third stage of hemostasis begins: the formation of a blood clot. First, blood changes from a liquid to a gel. At least 12 substances called clotting factors take part in a series of chemical reactions that eventually create a mesh of protein fibers within the blood. Each of the clotting factors has a very specific function. We will discuss just three of the substances here: prothrombin, thrombin, and fibrin. Prothrombin and fibrinogen are proteins that are produced and deposited in the blood by the liver.
ABO Group System[edit]The ABO blood group is represented by substances on the surface of red blood cells (RBCs). These substances are important because they contain specific sequences of amino acid and carbohydrates which are antigenic. As well as being on the surface of RBCs, some of these antigens are also present on the cells of other tissues. A complete blood type describes the set of 29 substances on the surface of RBCs, and an individual's blood type is one of the many possible combinations of blood group antigens. Usually only the ABO blood group system and the presence or absence of the Rhesus D antigen (also known as the Rhesus factor or RH factor) are determined and used to describe the blood type. Over 400 different blood group antigens have been found, many of these being very rare. If an individual is exposed to a blood group antigen that is not recognized as self, the individual can become sensitized to that antigen; the immune system makes specific antibodies which binds specifically to a particular blood group antigen and an immunological memory against that particular antigen is formed. These antibodies can bind to antigens on the surface of transfused red blood cells (or other tissue cells) often leading to destruction of the cells by recruitment of other components of the immune system. Knowledge of a individual's blood type is important to identify appropriate blood for transfusion or tissue for organ transplantation.
Surface Antigens[edit]Several different RBC surface antigens stemming from one allele (or very closely linked genes) are collectively labeled as a blood group system (or blood group). The two most important blood group systems were discovered during early experiments with blood transfusion, the ABO group in 1901 and the Rhesus group in 1937 . These two blood groups are reflected in the common nomenclature A positive, O negative, etc. with letters referring to the ABO group and positive/negative to the presence/absence of the RhD antigen of the Rhesus group. Development of the Coombs test in 1945 and the advent of transfusion medicine led to discovery of more blood groups.
Compatibility of blood typesBlood group AB individuals have both A and B antigens on the surface of their RBCs, and their blood serum does not contain any antibodies against either A or B antigen. Therefore, a individual with type AB blood can receive blood from any group (with AB being preferable), but can only donate blood to another group AB individual. AB blood is also known as "universal receiver".
Blood group A individuals have the A antigen on the surface of their RBCs, and blood serum containing IgM antibodies against the B antigen. Therefore, a group A individual can only receive blood from individuals of groups A or O (with A being preferable), and can donate blood to individuals of groups A or AB.
Blood group B individuals have the B antigen on their surface of their RBCs, and blood serum containing IgM antibodies against the A antigen. Therefore, a group B individual can only receive blood from individuals of groups B or O (with B being preferable), and can donate blood to individuals of groups B or AB.
Blood group O individuals do not have either A or B antigens on the surface of their RBCs, but their blood serum contains IgM antibodies against both A and B antigens. Therefore, a group O individual can only receive blood from a group O individual, but they can donate blood to individuals of any ABO blood group (ie A, B, O or AB). O blood is also known as "universal donor".
Inheritance[edit]Blood types are inherited and represent contributions from both parents. The ABO blood type is controlled by a single gene with three alleles: i, IA, and IB. The gene encodes an enzyme that modifies the carbohydrate content of the red blood cell antigens.
IA gives type A, IB gives type B, i give types O
Blood group inheritance
Mother/FatherOABAB
OOO, AO, BA, B
AO, AO, AO, A, B, ABA, B, AB
BO, BO, A, B, ABO, BA, B, AB
ABA, BA, B, ABA, B, ABA, B, ABIA and IB are dominant over i, so ii people have type O, IAIA or IAi have A, and IBIB or IBi have type B. IAIB people have both phenotypes because A and B are codominant, which means that type A and B parents can have an AB child. Thus, it is extremely unlikely for a type AB parent to have a type O child (it is not, however, direct proof of illegitimacy): the cis-AB phenotype has a single enzyme that creates both A and B antigens. The resulting red blood cells do not usually express A or B antigen at the same level that would be expected on common group A or B red blood cells, which can help solve the problem of an apparently genetically impossible blood group.
Rh Factor
Many people have the Rh Factor on the red blood cell. Rh carriers do not have the antibodies for the Rh Factor, but can make them if exposed to Rh. Most commonly Rh is seen when anti-Rh antibodies cross from the mothers placenta into the child before birth. The Rh Factor enters the child destroying the child's red blood cells. This is called Hemolytic Disease.
Compatibility in Blood/Plasma Transfusions[edit]Blood transfusions between donor and recipient of incompatible blood types can cause severe acute immunological reactions, hemolysis (RBCT destruction), renal failure, shock, and sometimes death. Antibodies can be highly active and can attack RBCs and bind components of the complement system to cause massive hemolysis of the transfused blood.
A patient should ideally receive their own blood or type-specific blood products to minimize the chance of a transfusion reaction. If time allows, the risk will further be reduced by cross-matching blood, in addition to blood typing both recipient and donor. Cross-matching involves mixing a sample of the recipient's blood with a sample of the donor's blood and checking to see if the mixture agglutinates, or forms clumps. Blood bank technicians usually check for agglutination with a microscope, and if it occurs, that particular donor's blood cannot be transfused to that particular recipient. Blood transfusion is a potentially risky medical procedure and it is vital that all blood specimens are correctly identified, so in cross-matching labeling is standardized using a barcode system known as ISBT 128.
Plasma compatibility tableDonorRecipient
OABAB
OOKOKOKOK
AOKOK
BOKOK
ABOKWhen considering a plasma transfusion, keep in mind that plasma carries antibodies and no antigens. For example you can't give type O plasma to a type A, B or AB, because a person with type O blood has A and B antibodies and the recipient would have an immune response. On the other hand an AB donor could give plasma to anyone, since they have no antibodies.
The table to the right is for plasma transfusions, and it's just the opposite for RBC transfusions. It doesn't take the Rh factor into effect, though, because most people don't have antibodies for the Rhesus factor (it only happens upon exposure).
Hemolytic Disease of the Newborn[edit]Often a pregnant woman carries a fetus with a different blood type to herself, and sometimes the mother forms antibodies against the red blood cells of the fetus, leading to low fetal blood counts, a condition known as hemolytic disease of the newborn.
Hemolytic disease of the newborn, (also known as HDN) is an alloimmune condition that develops in a fetus when the IgG antibodies produced by the mother and passing through the placenta include ones which attack the red blood cells in the fetal circulation. The red cells are broken down and the fetus can develop reticulocytosis and anemia. The fetal disease ranges from mild to very severe and fetal death from heart failure - hydrops fetalis - can occur. When the disease is moderate or severe many erythroblasts are present in the fetal blood and so these forms of the disease can be called erythroblastosis fetalis.
Before birth, options for treatment include intrauterine transfusion or early induction of labor when pulmonary maturity has been attained, fetal distress is present, or 35 to 37 weeks of gestation have passed. The mother may also undergo plasma exchange to reduce the circulating levels of antibody by as much as 75%.
After birth, treatment depends on the severity of the condition, but could include temperature stabilization and monitoring, phototherapy, transfusion with compatible packed red blood, exchange transfusion with a blood type compatible with both the infant and the mother, sodium bicarbonate for correction of acidosis and/or assisted ventilation.
Rh negative mothers who have had a pregnancy with or are pregnant with a Rh positive infant, are given Rh immune globulin (RhIG) also known as Rhogam, during pregnancy and after delivery to prevent sensitization to the D antigen. It works by binding any fetal red cells with the D antigen before the mother is able to produce an immune response and form anti-D IgG. A drawback to pre-partum administration of RhIG is that it causes a positive antibody screen when the mother is tested which is indistinguishable from immune reasons for antibody production.
Diseases of the Blood[edit]Von Willebrand Disease[edit]The most common inherited bleeding disorder, von Willebrand disease affects both men and women equally. Von Willebrand disease is similar to hemophilia in that it involves a definciency in the ability of blood to clot properly. Those affect by von Willebrand disease will have one or more of the following- low levels of von Willebrand factor (a protein that helps the blood to clot), and/or their von Willebrand factor doesn't work properly. While it is mostly an inherited disease (with factors contributed by both parents), von Willebrand disease may be an aquired syndrome in rare cases.
There are three types of von Willebrand disease: Type 1, which is the mildest and most common form of the disease; Type 2, which has four subtypes (2A, 2B, 2M, and 2N) and ranges from mild to moderate in severity; and finally, Type 3, which is very rare and is the most severe form.
Type 1In type 1 von Willebrand disease, there is a low level of von Willebrand factor. The level of factor VIII may also be lower than normal. This is the mildest and most common form of the disease. About 3 out of 4 people diagnosed with von Willebrand disease have type 1.
Type 2In type 2 von Willebrand disease, a defect in von Willebrand factor causes it to not work properly. Type 2 is divided into 2A, 2B, 2M, and 2N. Each is treated differently, so knowing the exact type is important.
People with type 1 and type 2 von Willebrand disease may have the following mild-to-moderate bleeding symptoms: easy bruising, nosebleeds, bleeding from the gums after a dental procedure, heavy menstrual bleeding in women, blood in their stools or urine (from bleeding in the intestines, stomach, kidneys or bladder), excessive bleeding after a cut or other accident or surgery.
Type 3People with type 3 von Willebrand disease usually have no von Willebrand factor and very low factor VIII. Type 3 is severe and very rare.
Symptoms of type 3 von Willebrand disease might include any of the symptoms of types 1 and 2, and also include severe bleeding episodes for no reason, which can be life threatening if not treated immediately. Bleeding into soft tissues or joints (hemarthrosis), causing severe pain and swelling, is another symptom.
TreatmentMany people with von Willebrand disease do not require treatment to manage the disease. However, if treatment is ncessary, it may include a range of different interventions depending on the severity. These involve medicine to increase the level of von Willebrand factor in the blood (DDAVP), medicine to prevent the breakdown of clots (called antifibrinolytic drugs), medicine to control heavy menstrual bleeding in women (often birth control pills), or injection of clotting factor concentrates (containing von Willebrand factor and factor VIII).
Disseminated Intravascular Coagulation[edit]Disseminated intravascular coagulation (DIC), also called consumptive coagulopathy, is a pathological process in the body where the blood starts to coagulate throughout the whole body. This depletes the body of its platelets and coagulation factors, and there is a paradoxically increased risk of hemorrhage. It occurs in critically ill patients, especially those with Gram-negative sepsis (particularly meningococcal sepsis) and acute promyelocytic leukemia.
Hemophilia[edit]Hemophilia is a disease where there is low or no blood protein, causing an inability to produce blood clots. There are two types of Hemophilia: Type A, which is a deficiency in factor VIII and Type B, (Christmas disease) a deficiency on factor IX. Because people with hemophilia have an impaired ability to make blood clots, even a little cut may take hours or days to fully clot, and a small bump or jar to the body could cause severe bruising that doesn't heal for months. Internal muscle bleeds are the most common symptom though, causing swelling and varying degrees of pain.
Hemophilia is passed down from mothers to their sons. Hemophilia is sometimes known as the "Royal Disease". This is because Queen Victoria, Queen of England (1837-1901), was a carrier of hemophilia. The hemophilia disease was passed down to her son Leopold who ended up dying at age 31. Queen Victoria also had two daughters who were carriers. These daughters passed hemophilia into the Spanish, German, and Russian royal families. One of the most famous stories is that of the Russian royal family. Alexandra, granddaughter to Queen Victoria, married Nicholas (Tsar of Russia in the 1900s). Alexandra was a carrier of the disease and passed the disease to their first son, Tsarevich Alexi, who was heir to the throne of Russia. The family tried to keep their son's secret from the people, but Alexi suffered with serious bruises and extreme pain. The family found help from a monk named Rasputin. He kept their secret and gained a great deal of power over the family, making them think he was their only hope. During this time of great turmoil in Russia, Nicholas and Alexandra spent most of their attentions on their son, and not on the people. It wasn't long before the Bolshevik Revolution of 1917 began.
Factor V Leiden[edit]The opposite of Hemophilia, Factor V Leiden is the name given to a variant of human factor V that causes a hypercoagulability disorder. In this disorder the Leiden variant of factor V, cannot be inactivated by activated protein C. Factor V Leiden is the most common hereditary hypercoagulability disorder amongst Eurasians. It is named after the city Leiden (The Netherlands), where it was first identified in 1994 by Prof R. Bertina et al. Those that have it are at a slightly higher risk of developing blood clots than those without. Those that test positive for factor V should avoid (oral contraceptives, obesity, smoking, and high blood pressure.)
Anemia[edit]Anemia (AmE) or anaemia (BrE), from the Greek (Ἀναιμία) meaning "without blood", refers to a deficiency of red blood cells (RBCs) and/or hemoglobin. This results in a reduced ability of blood to transfer oxygen to the tissues, causing hypoxia. Since all human cells depend on oxygen for survival, varying degrees of anemia can have a wide range of clinical consequences. Hemoglobin (the oxygen-carrying protein in the red blood cells) has to be present to ensure adequate oxygenation of all body tissues and organs.
The three main classes of anemia include excessive blood loss (acutely such as a hemorrhage or chronically through low-volume loss), excessive blood cell destruction (hemolysis) or deficient red blood cell production (ineffective hematopoiesis). In menstruating women, dietary iron deficiency is a common cause of deficient red blood cell production.
Sickle cell[edit]
Image of RBC's with Sickle Cell mutations.Sickle-cell disease is a general term for a group of genetic disorders caused by sickle hemoglobin (Hgb S or Hb S). In many forms of the disease, the red blood cells change shape upon deoxygenation because of polymerization of the abnormal sickle hemoglobin. This process damages the red blood cell membrane, and can cause the cells to become stuck in blood vessels. This deprives the downstream tissues of oxygen and causes ischemia and infarction. The disease is chronic and lifelong. Individuals are most often well, but their lives are punctuated by periodic painful attacks. In addition to periodic pain, there may be damage of internal organs, and/or stroke. Lifespan is often shortened with sufferers living to an average of 40 years. It is common in people from parts of the world where malaria is or was common, especially in sub-Saharan Africa or in descendants of those peoples.
Genetics: Sickle-cell disease is inherited in the autosomal recessive pattern, depicted above. The allele responsible for sickle cell anemia is autosomal recessive. A person who receives the defective gene from both father and mother develops the disease; a person who receives one defective and one healthy allele remains healthy, but can pass on the disease and is known as a carrier. If two parents who are carriers have a child, there is a 1-in-4 chance of their child developing the illness and a 1-in-2 chance of their child just being a carrier.
Polycythemia[edit]Polycythemia is a condition in which there is a net increase in the total circulating erythrocyte (red blood cell) mass of the body. There are several types of polycythemia.
Primary PolycythemiaIn primary polycythemia, there may be 8 to 9 million and occasionally 11 million erythrocytes per cubic millimeter of blood (a normal range for adults is 4-5 million), and the hematocrit may be as high as 70 to 80%. In addition, the total blood volume can increase to as much as twice as normal. The entire vascular system can become markedly engorged with blood, and circulation times for blood throughout the body can increase up to twice the normal value. The increased numbers of erythrocytes can increase of the viscosity of the blood to as much as five times normal. Capillaries can become plugged by the very viscous blood, and the flow of blood through the vessels tends to be extremely sluggish.
As a consequence of the above, people with untreated Polycythemia are at a risk of various thrombotic events (deep venous thrombosis, pulmonary embolism), heart attack and stroke, and have a substantial risk of Budd-Chiari syndrome (hepatic vein thrombosis). The condition is considered chronic; no cure exists. Symptomatic treatment (see below) can normalize the blood count and most patients can live a normal life for years.
Secondary polycythemiaSecondary polycythemia is caused by either appropriate or inappropriate increases in the production of erythropoietin that result in an increased production of erythrocytes. In secondary polycythemia, there may be 6 to 8 million and occasionally 9 million erythrocytes per cubic millimeter of blood. A type of secondary polycythemia in which the production of erythropoietin increases appropriately is called physiologic polycythemia. Physiologic polycythemia occurs in individuals living at high altitudes (4275 to 5200 meters), where oxygen availability is less than at sea level. Many athletes train at higher altitudes to take advantage of this effect — a legal form of blood doping. Actual polychthemia sufferers have been known to use their condition as an athletic advantage for greater stamina.
Other causes of secondary polycythemia include smoking, renal or liver tumors, or heart or lung diseases that result in hypoxia. Endocrine abnormalities, prominently including pheochromocytoma and adrenal adenoma with Cushing's Syndrome, are also secondary causes. Athletes and bodybuilders who abuse anabolic steroids or erythropoietin may develop secondary polycythemia.
Relative polycythemiaRelative polycythemia is an apparent rise of the erythrocyte level in the blood; however, the underlying cause is reduced blood plasma. Relative polycythemia is often caused by fluid loss i.e. burns, dehydration and stress polycythemia.
Leukemia[edit]Leukemia is a cancer of the blood or bone marrow characterized by an abnormal proliferation of blood cells, usually white blood cells (leukocytes). It is part of the broad group of diseases called hematological neoplasms. Damage to the bone marrow, by way of displacing the normal marrow cells with increasing numbers of malignant cells, results in a lack of blood platelets, which are important in the blood clotting process. This means people with leukemia may become bruised, bleed excessively, or develop pin-prick bleeds (petechiae).
White blood cells, which are involved in fighting pathogens, may be suppressed or dysfunctional, putting the patient at the risk of developing infections. The red blood cell deficiency leads to anaemia, which may cause dyspnea. All symptoms may also be attributable to other diseases; for diagnosis, blood tests and a bone marrow biopsy are required.
Shape
RBCS have a shape of a disk that appears to be “caved in” or almost flattened in the middle; this is called bi-concave. This bi-concave shape allows the RBC to carry oxygen and pass through even the smallest capillaries in the lungs. This shape also allows RBCs to stack like dinner plates and bend as they flow smoothly through the narrow blood vessels in the body. RBCs lack a nucleus (no DNA) and no organelles, meaning that these cells cannot divide or replicate themselves like the cells in our skin and muscles. RBCs have a short life span of about 120 days, however, as long as our myeloid tissue is working correctly, we will produce about 2-3 million RBCs per second. That is about 200 billion a day! This allows us to have more to replace the ones we lose.
Hemaglobin
The main component of the RBC is hemoglobin. Hemoglobin is protein that is composed of four protein subunits. Hemoglobin is responsible for the cell’s ability to transport oxygen and carbon dioxide. Hemoglobin, iron, and oxygen interact with each other, forming the RBCs' bright red color. You can call this interaction by product oxyhemoglobin. Carbon Monoxide binds with hemoglobin faster than oxygen, and stays bound for several hours, making hemoglobin temporarily unavailable for oxygen transport. One red blood cell contains about 200 million hemoglobin molecules. If all this hemoglobin was in the plasma rather than inside the cells, blood would be so "thick" that the heart would have a difficult time pumping it through. The thickness of blood is called viscosity. The greater the viscosity of blood, the more friction there is, and more pressure is needed to force blood through.
Functions[edit]The main function is the transportation of oxygen throughout the body and the ability of the blood to carry out carbon dioxide which is called carbamino – hemoglobin. Maintaining the balance of blood is important. The balance can be measured by the acid and base levels in the blood. This is called pH. Normal pH of blood ranges between 7.35-7.45; this normal blood is called Alkaline (less acidic then water). A drop in pH is called Acidic. This condition is also called Acidosis. A jump in pH higher then 7.45 is called "Alkalosis". To maintain the homeostasis (or balance,) the blood has tiny molecules within the RBC that help prevent drops or increases from happening.
From left to right diagram of Erythrocyte, Thrombocyte, and LeukocyteDestruction
Red blood cells are broken down and hemoglobin is released. The globin part of the hemoglobin is broken down into amino acid components, which in turn are recycled by the body. The iron is recovered and returned to the bone marrow to be reused. The heme portion of the molecule experiences a chemical change and then gets excreted as bile pigment (bilirubin) by the liver. Heme portion after being broken down contributes to the color of feces and your skin color changing after being bruised.
White Blood Cells[edit]Shape
White blood cells are different from red cells in the fact that they are usually larger in size 10-14 micrometers in diameter. White blood cells do not contain hemoglobin which in turn makes them translucent. Many times in diagrams or pictures white blood cells are represented in a blue color, mainly because blue is the color of the stain used to see the cells. White blood cells also have nucleii, that are some what segmented and are surrounded by electrons inside the membrane.
Functions[edit]White blood cells (leukocytes) are also known as "WBC's". White blood cells are made in the bone marrow but they also divide in the blood and lymphatic systems. They are commonly amoeboid (cells that move or feed by means of temporary projections, called pseudopods (false feet), and escape the circulatory system through the capillary beds. The different types of WBC's are Basophils, Eosinophils, Neutrophils, Monocytes, B- and T-cell lymphocytes. Neutrophils, Eosinophils, and Basophils are all granular leukocytes. Lymphocytes and Monocytes are agranular leukocytes. Basophils store and synthesize histamine which is important in allergic reactions. They enter the tissues and become "mast cells" which help blood flow to injured tissues by the release of histamine. Eosinophils are chemotoxic and kill parasites. Neutrophils are the first to act when there is an infection and are also the most abundant white blood cells. Neutrophils fight bacteria and viruses by phagocytosis which mean they engulf pathogens that may cause infection. The life span of a of Neutrophil is only about 12-48 hours. Monocytes are the biggest of the white blood cells and are responsible for rallying the cells to defend the body. Monocytes carry out phagocytosis and are also called macrophages. Lymphocytes help with our immune response. There are two Lymphocytes: the B- and T- cell. B-Lymphocytes produce antibodies that find and mark pathogens for destruction. T-Lymphocytes kill anything that they deem abnormal to the body.
WBCs are classified by phenotype which can be identified by looking at the WBCs under a microscope. The Granular phenotype are able to stain blue. The Agranular phenotype are able to stain red. Neutrophils make up 50-70% of Granular cells Eosinophils make up 2-4%, and Basophils 0-1%. Monocytes make up 2-8% of Agranular cells. B and T Lymphocytes make up 20-30%. As you can see, there is a great deal of differentiation between WBCs. These special cells help our bodies defend themselves against pathogens. Not only do they help our immune system but they remove toxins, wastes, and abnormal or damaged cells. Thus, we can say that WBCs' main function is being Phagocytic which means to engulf or swallow cells.
Platelets[edit]
A 250 ml bag of newly collected platelets.Platelets, also called thrombocytes, are membrane-bound cell fragments. Platelets have no nucleus, they are between one to two micrometers in diameter, and are about 1/10th to 1/20th as abundant as white blood cells. Less than 1% of whole blood consists of platelets. They result from fragmentation of large cells called Megakaryocytes - which are cells derived from stem cells in the bone marrow. Platelets are produced at a rate of 200 billion per day. Their production is regulated by the hormone called Thrombopoietin. The circulating life of a platelet is 8–10 days. The sticky surface of the platelets allow them to accumulate at the site of broken blood vessels to form a clot. This aids in the process of hemostasis ("blood stopping"). Platelets secrete factors that increase local platelet aggregation (e.g., Thromboxane A), enhance vasoconstriction (e.g., Serotonin), and promote blood coagulation (e.g., Thromboplastin).
Hemostasis (Coagulation or Clotting)[edit]Hemostasis is the natural process of stopping blood flow or loss of blood following an injury. (hemo = blood; stasis = standing). It has three stages: (1) vascular spasm, vasoconstriction, or intense contraction of blood vessels, (2) formation of a platelet plug and (3) blood clotting or coagulation. Once the flow of blood has been stopped, tissue repair can begin.
Vascular spasm or Vasoconsriction: In a normal individual, immediately after a blood vessel has been cut and endothelial cells are damaged, vasoconstriction occurs, thus slowing blood flow to the area. Smooth muscle in the vessel wall goes through spasms or intense contractions that constrict the vessel. If the vessels are small, spasms compress the inner walls together and may be able to stop the bleeding completely. If the vessels are medium to large-sized, the spasms slow down immediate outflow of blood, lessening the damage but still preparing the vessel for the later steps of hemostasis. These vascular spasms usually last for about 30 minutes, long enough for the next two stages of hemostasis to take place.
Formation of a Platelet Plug: Within 20 seconds of an injury, coagulation is initiated. Contrary to popular belief, clotting of a cut on the skin is not initiated by air or drying out, but by platelets adhering to and activated by collagen in the blood vessels endothelium. The activated platelets then release the contents of their granules, which contain a variety of substances that stimulate further platelet activation and enhance the hemostatic process.
When the lining of a blood vessel breaks and endothelial cells are damaged, revealing collagen proteins in the vessel wall, platelets swell, grow spiky extensions, and start clumping together. They start to stick to each other and the walls of the vessel. This continues as more platelets congregate and undergo these same transformations. This process results in a platelet plug that seals the injured area. If the injury is small, a platelet plug may be able to form and close it within several seconds. If the damage is more serious, the next step of blood clotting will take place. Platelets contain secretory granules. When they stick to the proteins in the vessel walls, they degranulate, thus releasing their products, which include ADP (adenosine diphosphate), serotonin, and thromboxane A2.
A Blood Clot Forms: If the platelet plug is not enough to stop the bleeding, the third stage of hemostasis begins: the formation of a blood clot. First, blood changes from a liquid to a gel. At least 12 substances called clotting factors take part in a series of chemical reactions that eventually create a mesh of protein fibers within the blood. Each of the clotting factors has a very specific function. We will discuss just three of the substances here: prothrombin, thrombin, and fibrin. Prothrombin and fibrinogen are proteins that are produced and deposited in the blood by the liver.
- Prothrombin: When blood vessels are damaged, vessels and nearby platelets are stimulated to release a substance called prothrombin activator, which in turn activates the conversion of prothrombin, a plasma protein, into an enzyme called thrombin. This reaction requires calcium ions.
- Thrombin: Thrombin facilitates the conversion of a soluble plasma protein called fibrinogen into long insoluble fibers or threads of the protein fibrin.
- Fibrin: Fibrinogen is cleaved by thrombin to form its active form, "fibrin." Fibrin threads wind around the platelet plug at the damaged area of the blood vessel, forming an interlocking network of fibers and a framework for the clot. This net of fibers traps and helps hold platelets, blood cells and other molecules tight to the site of injury, functioning as the initial clot. This temporary fibrin clot can form in less than a minute, and usually does a good job of reducing the blood flow. Next, platelets in the clot begin to shrink, tightening the clot and drawing together the vessel walls. Usually, this whole process of clot formation and tightening takes less than a half hour.
ABO Group System[edit]The ABO blood group is represented by substances on the surface of red blood cells (RBCs). These substances are important because they contain specific sequences of amino acid and carbohydrates which are antigenic. As well as being on the surface of RBCs, some of these antigens are also present on the cells of other tissues. A complete blood type describes the set of 29 substances on the surface of RBCs, and an individual's blood type is one of the many possible combinations of blood group antigens. Usually only the ABO blood group system and the presence or absence of the Rhesus D antigen (also known as the Rhesus factor or RH factor) are determined and used to describe the blood type. Over 400 different blood group antigens have been found, many of these being very rare. If an individual is exposed to a blood group antigen that is not recognized as self, the individual can become sensitized to that antigen; the immune system makes specific antibodies which binds specifically to a particular blood group antigen and an immunological memory against that particular antigen is formed. These antibodies can bind to antigens on the surface of transfused red blood cells (or other tissue cells) often leading to destruction of the cells by recruitment of other components of the immune system. Knowledge of a individual's blood type is important to identify appropriate blood for transfusion or tissue for organ transplantation.
Surface Antigens[edit]Several different RBC surface antigens stemming from one allele (or very closely linked genes) are collectively labeled as a blood group system (or blood group). The two most important blood group systems were discovered during early experiments with blood transfusion, the ABO group in 1901 and the Rhesus group in 1937 . These two blood groups are reflected in the common nomenclature A positive, O negative, etc. with letters referring to the ABO group and positive/negative to the presence/absence of the RhD antigen of the Rhesus group. Development of the Coombs test in 1945 and the advent of transfusion medicine led to discovery of more blood groups.
Compatibility of blood typesBlood group AB individuals have both A and B antigens on the surface of their RBCs, and their blood serum does not contain any antibodies against either A or B antigen. Therefore, a individual with type AB blood can receive blood from any group (with AB being preferable), but can only donate blood to another group AB individual. AB blood is also known as "universal receiver".
Blood group A individuals have the A antigen on the surface of their RBCs, and blood serum containing IgM antibodies against the B antigen. Therefore, a group A individual can only receive blood from individuals of groups A or O (with A being preferable), and can donate blood to individuals of groups A or AB.
Blood group B individuals have the B antigen on their surface of their RBCs, and blood serum containing IgM antibodies against the A antigen. Therefore, a group B individual can only receive blood from individuals of groups B or O (with B being preferable), and can donate blood to individuals of groups B or AB.
Blood group O individuals do not have either A or B antigens on the surface of their RBCs, but their blood serum contains IgM antibodies against both A and B antigens. Therefore, a group O individual can only receive blood from a group O individual, but they can donate blood to individuals of any ABO blood group (ie A, B, O or AB). O blood is also known as "universal donor".
Inheritance[edit]Blood types are inherited and represent contributions from both parents. The ABO blood type is controlled by a single gene with three alleles: i, IA, and IB. The gene encodes an enzyme that modifies the carbohydrate content of the red blood cell antigens.
IA gives type A, IB gives type B, i give types O
Blood group inheritance
Mother/FatherOABAB
OOO, AO, BA, B
AO, AO, AO, A, B, ABA, B, AB
BO, BO, A, B, ABO, BA, B, AB
ABA, BA, B, ABA, B, ABA, B, ABIA and IB are dominant over i, so ii people have type O, IAIA or IAi have A, and IBIB or IBi have type B. IAIB people have both phenotypes because A and B are codominant, which means that type A and B parents can have an AB child. Thus, it is extremely unlikely for a type AB parent to have a type O child (it is not, however, direct proof of illegitimacy): the cis-AB phenotype has a single enzyme that creates both A and B antigens. The resulting red blood cells do not usually express A or B antigen at the same level that would be expected on common group A or B red blood cells, which can help solve the problem of an apparently genetically impossible blood group.
Rh Factor
Many people have the Rh Factor on the red blood cell. Rh carriers do not have the antibodies for the Rh Factor, but can make them if exposed to Rh. Most commonly Rh is seen when anti-Rh antibodies cross from the mothers placenta into the child before birth. The Rh Factor enters the child destroying the child's red blood cells. This is called Hemolytic Disease.
Compatibility in Blood/Plasma Transfusions[edit]Blood transfusions between donor and recipient of incompatible blood types can cause severe acute immunological reactions, hemolysis (RBCT destruction), renal failure, shock, and sometimes death. Antibodies can be highly active and can attack RBCs and bind components of the complement system to cause massive hemolysis of the transfused blood.
A patient should ideally receive their own blood or type-specific blood products to minimize the chance of a transfusion reaction. If time allows, the risk will further be reduced by cross-matching blood, in addition to blood typing both recipient and donor. Cross-matching involves mixing a sample of the recipient's blood with a sample of the donor's blood and checking to see if the mixture agglutinates, or forms clumps. Blood bank technicians usually check for agglutination with a microscope, and if it occurs, that particular donor's blood cannot be transfused to that particular recipient. Blood transfusion is a potentially risky medical procedure and it is vital that all blood specimens are correctly identified, so in cross-matching labeling is standardized using a barcode system known as ISBT 128.
Plasma compatibility tableDonorRecipient
OABAB
OOKOKOKOK
AOKOK
BOKOK
ABOKWhen considering a plasma transfusion, keep in mind that plasma carries antibodies and no antigens. For example you can't give type O plasma to a type A, B or AB, because a person with type O blood has A and B antibodies and the recipient would have an immune response. On the other hand an AB donor could give plasma to anyone, since they have no antibodies.
The table to the right is for plasma transfusions, and it's just the opposite for RBC transfusions. It doesn't take the Rh factor into effect, though, because most people don't have antibodies for the Rhesus factor (it only happens upon exposure).
Hemolytic Disease of the Newborn[edit]Often a pregnant woman carries a fetus with a different blood type to herself, and sometimes the mother forms antibodies against the red blood cells of the fetus, leading to low fetal blood counts, a condition known as hemolytic disease of the newborn.
Hemolytic disease of the newborn, (also known as HDN) is an alloimmune condition that develops in a fetus when the IgG antibodies produced by the mother and passing through the placenta include ones which attack the red blood cells in the fetal circulation. The red cells are broken down and the fetus can develop reticulocytosis and anemia. The fetal disease ranges from mild to very severe and fetal death from heart failure - hydrops fetalis - can occur. When the disease is moderate or severe many erythroblasts are present in the fetal blood and so these forms of the disease can be called erythroblastosis fetalis.
Before birth, options for treatment include intrauterine transfusion or early induction of labor when pulmonary maturity has been attained, fetal distress is present, or 35 to 37 weeks of gestation have passed. The mother may also undergo plasma exchange to reduce the circulating levels of antibody by as much as 75%.
After birth, treatment depends on the severity of the condition, but could include temperature stabilization and monitoring, phototherapy, transfusion with compatible packed red blood, exchange transfusion with a blood type compatible with both the infant and the mother, sodium bicarbonate for correction of acidosis and/or assisted ventilation.
Rh negative mothers who have had a pregnancy with or are pregnant with a Rh positive infant, are given Rh immune globulin (RhIG) also known as Rhogam, during pregnancy and after delivery to prevent sensitization to the D antigen. It works by binding any fetal red cells with the D antigen before the mother is able to produce an immune response and form anti-D IgG. A drawback to pre-partum administration of RhIG is that it causes a positive antibody screen when the mother is tested which is indistinguishable from immune reasons for antibody production.
Diseases of the Blood[edit]Von Willebrand Disease[edit]The most common inherited bleeding disorder, von Willebrand disease affects both men and women equally. Von Willebrand disease is similar to hemophilia in that it involves a definciency in the ability of blood to clot properly. Those affect by von Willebrand disease will have one or more of the following- low levels of von Willebrand factor (a protein that helps the blood to clot), and/or their von Willebrand factor doesn't work properly. While it is mostly an inherited disease (with factors contributed by both parents), von Willebrand disease may be an aquired syndrome in rare cases.
There are three types of von Willebrand disease: Type 1, which is the mildest and most common form of the disease; Type 2, which has four subtypes (2A, 2B, 2M, and 2N) and ranges from mild to moderate in severity; and finally, Type 3, which is very rare and is the most severe form.
Type 1In type 1 von Willebrand disease, there is a low level of von Willebrand factor. The level of factor VIII may also be lower than normal. This is the mildest and most common form of the disease. About 3 out of 4 people diagnosed with von Willebrand disease have type 1.
Type 2In type 2 von Willebrand disease, a defect in von Willebrand factor causes it to not work properly. Type 2 is divided into 2A, 2B, 2M, and 2N. Each is treated differently, so knowing the exact type is important.
People with type 1 and type 2 von Willebrand disease may have the following mild-to-moderate bleeding symptoms: easy bruising, nosebleeds, bleeding from the gums after a dental procedure, heavy menstrual bleeding in women, blood in their stools or urine (from bleeding in the intestines, stomach, kidneys or bladder), excessive bleeding after a cut or other accident or surgery.
Type 3People with type 3 von Willebrand disease usually have no von Willebrand factor and very low factor VIII. Type 3 is severe and very rare.
Symptoms of type 3 von Willebrand disease might include any of the symptoms of types 1 and 2, and also include severe bleeding episodes for no reason, which can be life threatening if not treated immediately. Bleeding into soft tissues or joints (hemarthrosis), causing severe pain and swelling, is another symptom.
TreatmentMany people with von Willebrand disease do not require treatment to manage the disease. However, if treatment is ncessary, it may include a range of different interventions depending on the severity. These involve medicine to increase the level of von Willebrand factor in the blood (DDAVP), medicine to prevent the breakdown of clots (called antifibrinolytic drugs), medicine to control heavy menstrual bleeding in women (often birth control pills), or injection of clotting factor concentrates (containing von Willebrand factor and factor VIII).
Disseminated Intravascular Coagulation[edit]Disseminated intravascular coagulation (DIC), also called consumptive coagulopathy, is a pathological process in the body where the blood starts to coagulate throughout the whole body. This depletes the body of its platelets and coagulation factors, and there is a paradoxically increased risk of hemorrhage. It occurs in critically ill patients, especially those with Gram-negative sepsis (particularly meningococcal sepsis) and acute promyelocytic leukemia.
Hemophilia[edit]Hemophilia is a disease where there is low or no blood protein, causing an inability to produce blood clots. There are two types of Hemophilia: Type A, which is a deficiency in factor VIII and Type B, (Christmas disease) a deficiency on factor IX. Because people with hemophilia have an impaired ability to make blood clots, even a little cut may take hours or days to fully clot, and a small bump or jar to the body could cause severe bruising that doesn't heal for months. Internal muscle bleeds are the most common symptom though, causing swelling and varying degrees of pain.
Hemophilia is passed down from mothers to their sons. Hemophilia is sometimes known as the "Royal Disease". This is because Queen Victoria, Queen of England (1837-1901), was a carrier of hemophilia. The hemophilia disease was passed down to her son Leopold who ended up dying at age 31. Queen Victoria also had two daughters who were carriers. These daughters passed hemophilia into the Spanish, German, and Russian royal families. One of the most famous stories is that of the Russian royal family. Alexandra, granddaughter to Queen Victoria, married Nicholas (Tsar of Russia in the 1900s). Alexandra was a carrier of the disease and passed the disease to their first son, Tsarevich Alexi, who was heir to the throne of Russia. The family tried to keep their son's secret from the people, but Alexi suffered with serious bruises and extreme pain. The family found help from a monk named Rasputin. He kept their secret and gained a great deal of power over the family, making them think he was their only hope. During this time of great turmoil in Russia, Nicholas and Alexandra spent most of their attentions on their son, and not on the people. It wasn't long before the Bolshevik Revolution of 1917 began.
Factor V Leiden[edit]The opposite of Hemophilia, Factor V Leiden is the name given to a variant of human factor V that causes a hypercoagulability disorder. In this disorder the Leiden variant of factor V, cannot be inactivated by activated protein C. Factor V Leiden is the most common hereditary hypercoagulability disorder amongst Eurasians. It is named after the city Leiden (The Netherlands), where it was first identified in 1994 by Prof R. Bertina et al. Those that have it are at a slightly higher risk of developing blood clots than those without. Those that test positive for factor V should avoid (oral contraceptives, obesity, smoking, and high blood pressure.)
Anemia[edit]Anemia (AmE) or anaemia (BrE), from the Greek (Ἀναιμία) meaning "without blood", refers to a deficiency of red blood cells (RBCs) and/or hemoglobin. This results in a reduced ability of blood to transfer oxygen to the tissues, causing hypoxia. Since all human cells depend on oxygen for survival, varying degrees of anemia can have a wide range of clinical consequences. Hemoglobin (the oxygen-carrying protein in the red blood cells) has to be present to ensure adequate oxygenation of all body tissues and organs.
The three main classes of anemia include excessive blood loss (acutely such as a hemorrhage or chronically through low-volume loss), excessive blood cell destruction (hemolysis) or deficient red blood cell production (ineffective hematopoiesis). In menstruating women, dietary iron deficiency is a common cause of deficient red blood cell production.
Sickle cell[edit]
Image of RBC's with Sickle Cell mutations.Sickle-cell disease is a general term for a group of genetic disorders caused by sickle hemoglobin (Hgb S or Hb S). In many forms of the disease, the red blood cells change shape upon deoxygenation because of polymerization of the abnormal sickle hemoglobin. This process damages the red blood cell membrane, and can cause the cells to become stuck in blood vessels. This deprives the downstream tissues of oxygen and causes ischemia and infarction. The disease is chronic and lifelong. Individuals are most often well, but their lives are punctuated by periodic painful attacks. In addition to periodic pain, there may be damage of internal organs, and/or stroke. Lifespan is often shortened with sufferers living to an average of 40 years. It is common in people from parts of the world where malaria is or was common, especially in sub-Saharan Africa or in descendants of those peoples.
Genetics: Sickle-cell disease is inherited in the autosomal recessive pattern, depicted above. The allele responsible for sickle cell anemia is autosomal recessive. A person who receives the defective gene from both father and mother develops the disease; a person who receives one defective and one healthy allele remains healthy, but can pass on the disease and is known as a carrier. If two parents who are carriers have a child, there is a 1-in-4 chance of their child developing the illness and a 1-in-2 chance of their child just being a carrier.
Polycythemia[edit]Polycythemia is a condition in which there is a net increase in the total circulating erythrocyte (red blood cell) mass of the body. There are several types of polycythemia.
Primary PolycythemiaIn primary polycythemia, there may be 8 to 9 million and occasionally 11 million erythrocytes per cubic millimeter of blood (a normal range for adults is 4-5 million), and the hematocrit may be as high as 70 to 80%. In addition, the total blood volume can increase to as much as twice as normal. The entire vascular system can become markedly engorged with blood, and circulation times for blood throughout the body can increase up to twice the normal value. The increased numbers of erythrocytes can increase of the viscosity of the blood to as much as five times normal. Capillaries can become plugged by the very viscous blood, and the flow of blood through the vessels tends to be extremely sluggish.
As a consequence of the above, people with untreated Polycythemia are at a risk of various thrombotic events (deep venous thrombosis, pulmonary embolism), heart attack and stroke, and have a substantial risk of Budd-Chiari syndrome (hepatic vein thrombosis). The condition is considered chronic; no cure exists. Symptomatic treatment (see below) can normalize the blood count and most patients can live a normal life for years.
Secondary polycythemiaSecondary polycythemia is caused by either appropriate or inappropriate increases in the production of erythropoietin that result in an increased production of erythrocytes. In secondary polycythemia, there may be 6 to 8 million and occasionally 9 million erythrocytes per cubic millimeter of blood. A type of secondary polycythemia in which the production of erythropoietin increases appropriately is called physiologic polycythemia. Physiologic polycythemia occurs in individuals living at high altitudes (4275 to 5200 meters), where oxygen availability is less than at sea level. Many athletes train at higher altitudes to take advantage of this effect — a legal form of blood doping. Actual polychthemia sufferers have been known to use their condition as an athletic advantage for greater stamina.
Other causes of secondary polycythemia include smoking, renal or liver tumors, or heart or lung diseases that result in hypoxia. Endocrine abnormalities, prominently including pheochromocytoma and adrenal adenoma with Cushing's Syndrome, are also secondary causes. Athletes and bodybuilders who abuse anabolic steroids or erythropoietin may develop secondary polycythemia.
Relative polycythemiaRelative polycythemia is an apparent rise of the erythrocyte level in the blood; however, the underlying cause is reduced blood plasma. Relative polycythemia is often caused by fluid loss i.e. burns, dehydration and stress polycythemia.
Leukemia[edit]Leukemia is a cancer of the blood or bone marrow characterized by an abnormal proliferation of blood cells, usually white blood cells (leukocytes). It is part of the broad group of diseases called hematological neoplasms. Damage to the bone marrow, by way of displacing the normal marrow cells with increasing numbers of malignant cells, results in a lack of blood platelets, which are important in the blood clotting process. This means people with leukemia may become bruised, bleed excessively, or develop pin-prick bleeds (petechiae).
White blood cells, which are involved in fighting pathogens, may be suppressed or dysfunctional, putting the patient at the risk of developing infections. The red blood cell deficiency leads to anaemia, which may cause dyspnea. All symptoms may also be attributable to other diseases; for diagnosis, blood tests and a bone marrow biopsy are required.
Proper physiological functioning depends on a very tight balance between the concentrations of acids and bases in the blood. Acid-balance balance is measured using the pH scale. A variety of buffering systems permits blood and other bodily fluids to maintain a narrow pH range, even in the face of perturbations. A buffer is a chemical system that prevents a radical change in fluid pH by dampening the change in hydrogen ion concentrations in the case of excess acid or base. Most commonly, the substance that absorbs the ions is either a weak acid, which takes up hydroxyl ions, or a weak base, which takes up hydrogen ions.
The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The kidneys help control acid-base balance by excreting hydrogen ions and generating bicarbonate that helps maintain blood plasma pH within a normal range. Protein buffer systems work predominantly inside cells.
Protein Buffers in Blood Plasma and Cells
Nearly all proteins can function as buffers. Proteins are made up of amino acids, which contain positively charged amino groups and negatively charged carboxyl groups. The charged regions of these molecules can bind hydrogen and hydroxyl ions, and thus function as buffers. Buffering by proteins accounts for two-thirds of the buffering power of the blood and most of the buffering within cells.
Hemoglobin as a Buffer
Hemoglobin is the principal protein inside of red blood cells and accounts for one-third of the mass of the cell. During the conversion of CO2 into bicarbonate, hydrogen ions liberated in the reaction are buffered by hemoglobin, which is reduced by the dissociation of oxygen. This buffering helps maintain normal pH. The process is reversed in the pulmonary capillaries to re-form CO2, which then can diffuse into the air sacs to be exhaled into the atmosphere.
Phosphate Buffer
Phosphates are found in the blood in two forms: sodium dihydrogen phosphate (Na2H2PO4−), which is a weak acid, and sodium monohydrogen phosphate (Na2HPO42-), which is a weak base. When Na2HPO42- comes into contact with a strong acid, such as HCl, the base picks up a second hydrogen ion to form the weak acid Na2H2PO4− and sodium chloride, NaCl. When Na2HPO42− (the weak acid) comes into contact with a strong base, such as sodium hydroxide (NaOH), the weak acid reverts back to the weak base and produces water. Acids and bases are still present, but they hold onto the ions.
HCl + Na2HPO4→NaH2PO4 + NaCl
(strong acid) + (weak base) → (weak acid) + (salt)
NaOH + NaH2PO4→Na2HPO4 + H2O
(strong base) + (weak acid) → (weak base) + (water)
Bicarbonate-Carbonic Acid Buffer
The bicarbonate-carbonic acid buffer works in a fashion similar to phosphate buffers. The bicarbonate is regulated in the blood by sodium, as are the phosphate ions.
(sodium bicarbonate) + (strong acid) → (weak acid) + (salt)
H2CO3 + NaOH→HCO3- + H2O
(weak acid) + (strong base)→(bicarbonate) + (water)
As with the phosphate buffer, a weak acid or weak base captures the free ions, and a significant change in pH is prevented. Bicarbonate ions and carbonic acid are present in the blood in a 20:1 ratio if the blood pH is within the normal range. With 20 times more bicarbonate than carbonic acid, this capture system is most efficient at buffering changes that would make the blood more acidic. This is useful because most of the body’s metabolic wastes, such as lactic acid and ketones, are acids.
Carbonic acid levels in the blood are controlled by the expiration of CO2 through the lungs. In red blood cells, carbonic anhydrase forces the dissociation of the acid, rendering the blood less acidic. Because of this acid dissociation, CO2 is exhaled (see equations above). The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are conserved and passed back into the blood. However, the bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body.
Respiratory Regulation of Acid-Base Balance
The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (Figure 2). CO2 in the blood readily reacts with water to form carbonic acid, and the levels of CO2 and carbonic acid in the blood are in equilibrium. When the CO2 level in the blood rises (as it does when you hold your breath), the excess CO2 reacts with water to form additional carbonic acid, lowering blood pH. Increasing the rate and/or depth of respiration (which you might feel the “urge” to do after holding your breath) allows you to exhale more CO2. The loss of CO2 from the body reduces blood levels of carbonic acid and thereby adjusts the pH upward, toward normal levels. As you might have surmised, this process also works in the opposite direction. Excessive deep and rapid breathing (as in hyperventilation) rids the blood of CO2 and reduces the level of carbonic acid, making the blood too alkaline. This brief alkalosis can be remedied by rebreathing air that has been exhaled into a paper bag. Rebreathing exhaled air will rapidly bring blood pH down toward normal.
Respiratory Regulation of Blood pH. The respiratory system can reduce blood pH by removing CO2 from the blood.The chemical reactions that regulate the levels of CO2 and carbonic acid occur in the lungs when blood travels through the lung’s pulmonary capillaries. Minor adjustments in breathing are usually sufficient to adjust the pH of the blood by changing how much CO2 is exhaled. In fact, doubling the respiratory rate for less than 1 minute, removing “extra” CO2, would increase the blood pH by 0.2. This situation is common if you are exercising strenuously over a period of time. To keep up the necessary energy production, you would produce excess CO2 (and lactic acid if exercising beyond your aerobic threshold). In order to balance the increased acid production, the respiration rate goes up to remove the CO2. This helps to keep you from developing acidosis.
The body regulates the respiratory rate by the use of chemoreceptors, which primarily use CO2 as a signal. Peripheral blood sensors are found in the walls of the aorta and carotid arteries. These sensors signal the brain to provide immediate adjustments to the respiratory rate if CO2 levels rise or fall. Yet other sensors are found in the brain itself. Changes in the pH of CSF affect the respiratory center in the medulla oblongata, which can directly modulate breathing rate to bring the pH back into the normal range.
Hypercapnia, or abnormally elevated blood levels of CO2, occurs in any situation that impairs respiratory functions, including pneumonia and congestive heart failure. Reduced breathing (hypoventilation) due to drugs such as morphine, barbiturates, or ethanol (or even just holding one’s breath) can also result in hypercapnia. Hypocapnia, or abnormally low blood levels of CO2, occurs with any cause of hyperventilation that drives off the CO2, such as salicylate toxicity, elevated room temperatures, fever, or hysteria.
Renal Regulation of Acid-Base Balance
The renal regulation of the body’s acid-base balance addresses the metabolic component of the buffering system. Whereas the respiratory system (together with breathing centers in the brain) controls the blood levels of carbonic acid by controlling the exhalation of CO2, the renal system controls the blood levels of bicarbonate. A decrease of blood bicarbonate can result from the inhibition of carbonic anhydrase by certain diuretics or from excessive bicarbonate loss due to diarrhea. Blood bicarbonate levels are also typically lower in people who have Addison’s disease (chronic adrenal insufficiency), in which aldosterone levels are reduced, and in people who have renal damage, such as chronic nephritis. Finally, low bicarbonate blood levels can result from elevated levels of ketones (common in unmanaged diabetes mellitus), which bind bicarbonate in the filtrate and prevent its conservation.
Bicarbonate ions, HCO3–, found in the filtrate, are essential to the bicarbonate buffer system, yet the cells of the tubule are not permeable to bicarbonate ions. The steps involved in supplying bicarbonate ions to the system are seen in Figure 3 and are summarized below:
Conservation of Bicarbonate in the Kidney. Tubular cells are not permeable to bicarbonate; thus, bicarbonate is conserved rather than reabsorbed. Steps 1 and 2 of bicarbonate conservation are indicated.It is also possible that salts in the filtrate, such as sulfates, phosphates, or ammonia, will capture hydrogen ions. If this occurs, the hydrogen ions will not be available to combine with bicarbonate ions and produce CO2. In such cases, bicarbonate ions are not conserved from the filtrate to the blood, which will also contribute to a pH imbalance and acidosis.
The hydrogen ions also compete with potassium to exchange with sodium in the renal tubules. If more potassium is present than normal, potassium, rather than the hydrogen ions, will be exchanged, and increased potassium enters the filtrate. When this occurs, fewer hydrogen ions in the filtrate participate in the conversion of bicarbonate into CO2 and less bicarbonate is conserved. If there is less potassium, more hydrogen ions enter the filtrate to be exchanged with sodium and more bicarbonate is conserved.
Chloride ions are important in neutralizing positive ion charges in the body. If chloride is lost, the body uses bicarbonate ions in place of the lost chloride ions. Thus, lost chloride results in an increased reabsorption of bicarbonate by the renal system.
Disorders of the…Acid-Base Balance: Ketoacidosis
Diabetic acidosis, or ketoacidosis, occurs most frequently in people with poorly controlled diabetes mellitus. When certain tissues in the body cannot get adequate amounts of glucose, they depend on the breakdown of fatty acids for energy. When acetyl groups break off the fatty acid chains, the acetyl groups then non-enzymatically combine to form ketone bodies, acetoacetic acid, beta-hydroxybutyric acid, and acetone, all of which increase the acidity of the blood. In this condition, the brain isn’t supplied with enough of its fuel—glucose—to produce all of the ATP it requires to function.
Ketoacidosis can be severe and, if not detected and treated properly, can lead to diabetic coma, which can be fatal. A common early symptom of ketoacidosis is deep, rapid breathing as the body attempts to drive off CO2 and compensate for the acidosis. Another common symptom is fruity-smelling breath, due to the exhalation of acetone. Other symptoms include dry skin and mouth, a flushed face, nausea, vomiting, and stomach pain. Treatment for diabetic coma is ingestion or injection of sugar; its prevention is the proper daily administration of insulin.
A person who is diabetic and uses insulin can initiate ketoacidosis if a dose of insulin is missed. Among people with type 2 diabetes, those of Hispanic and African-American descent are more likely to go into ketoacidosis than those of other ethnic backgrounds, although the reason for this is unknown.
Protein Buffers in Blood Plasma and Cells
Nearly all proteins can function as buffers. Proteins are made up of amino acids, which contain positively charged amino groups and negatively charged carboxyl groups. The charged regions of these molecules can bind hydrogen and hydroxyl ions, and thus function as buffers. Buffering by proteins accounts for two-thirds of the buffering power of the blood and most of the buffering within cells.
Hemoglobin as a Buffer
Hemoglobin is the principal protein inside of red blood cells and accounts for one-third of the mass of the cell. During the conversion of CO2 into bicarbonate, hydrogen ions liberated in the reaction are buffered by hemoglobin, which is reduced by the dissociation of oxygen. This buffering helps maintain normal pH. The process is reversed in the pulmonary capillaries to re-form CO2, which then can diffuse into the air sacs to be exhaled into the atmosphere.
Phosphate Buffer
Phosphates are found in the blood in two forms: sodium dihydrogen phosphate (Na2H2PO4−), which is a weak acid, and sodium monohydrogen phosphate (Na2HPO42-), which is a weak base. When Na2HPO42- comes into contact with a strong acid, such as HCl, the base picks up a second hydrogen ion to form the weak acid Na2H2PO4− and sodium chloride, NaCl. When Na2HPO42− (the weak acid) comes into contact with a strong base, such as sodium hydroxide (NaOH), the weak acid reverts back to the weak base and produces water. Acids and bases are still present, but they hold onto the ions.
HCl + Na2HPO4→NaH2PO4 + NaCl
(strong acid) + (weak base) → (weak acid) + (salt)
NaOH + NaH2PO4→Na2HPO4 + H2O
(strong base) + (weak acid) → (weak base) + (water)
Bicarbonate-Carbonic Acid Buffer
The bicarbonate-carbonic acid buffer works in a fashion similar to phosphate buffers. The bicarbonate is regulated in the blood by sodium, as are the phosphate ions.
- When sodium bicarbonate (NaHCO3), comes into contact with a strong acid, such as HCl, carbonic acid (H2CO3), which is a weak acid, and NaCl are formed.
- When carbonic acid comes into contact with a strong base, such as NaOH, bicarbonate and water are formed.
(sodium bicarbonate) + (strong acid) → (weak acid) + (salt)
H2CO3 + NaOH→HCO3- + H2O
(weak acid) + (strong base)→(bicarbonate) + (water)
As with the phosphate buffer, a weak acid or weak base captures the free ions, and a significant change in pH is prevented. Bicarbonate ions and carbonic acid are present in the blood in a 20:1 ratio if the blood pH is within the normal range. With 20 times more bicarbonate than carbonic acid, this capture system is most efficient at buffering changes that would make the blood more acidic. This is useful because most of the body’s metabolic wastes, such as lactic acid and ketones, are acids.
Carbonic acid levels in the blood are controlled by the expiration of CO2 through the lungs. In red blood cells, carbonic anhydrase forces the dissociation of the acid, rendering the blood less acidic. Because of this acid dissociation, CO2 is exhaled (see equations above). The level of bicarbonate in the blood is controlled through the renal system, where bicarbonate ions in the renal filtrate are conserved and passed back into the blood. However, the bicarbonate buffer is the primary buffering system of the IF surrounding the cells in tissues throughout the body.
Respiratory Regulation of Acid-Base Balance
The respiratory system contributes to the balance of acids and bases in the body by regulating the blood levels of carbonic acid (Figure 2). CO2 in the blood readily reacts with water to form carbonic acid, and the levels of CO2 and carbonic acid in the blood are in equilibrium. When the CO2 level in the blood rises (as it does when you hold your breath), the excess CO2 reacts with water to form additional carbonic acid, lowering blood pH. Increasing the rate and/or depth of respiration (which you might feel the “urge” to do after holding your breath) allows you to exhale more CO2. The loss of CO2 from the body reduces blood levels of carbonic acid and thereby adjusts the pH upward, toward normal levels. As you might have surmised, this process also works in the opposite direction. Excessive deep and rapid breathing (as in hyperventilation) rids the blood of CO2 and reduces the level of carbonic acid, making the blood too alkaline. This brief alkalosis can be remedied by rebreathing air that has been exhaled into a paper bag. Rebreathing exhaled air will rapidly bring blood pH down toward normal.
Respiratory Regulation of Blood pH. The respiratory system can reduce blood pH by removing CO2 from the blood.The chemical reactions that regulate the levels of CO2 and carbonic acid occur in the lungs when blood travels through the lung’s pulmonary capillaries. Minor adjustments in breathing are usually sufficient to adjust the pH of the blood by changing how much CO2 is exhaled. In fact, doubling the respiratory rate for less than 1 minute, removing “extra” CO2, would increase the blood pH by 0.2. This situation is common if you are exercising strenuously over a period of time. To keep up the necessary energy production, you would produce excess CO2 (and lactic acid if exercising beyond your aerobic threshold). In order to balance the increased acid production, the respiration rate goes up to remove the CO2. This helps to keep you from developing acidosis.
The body regulates the respiratory rate by the use of chemoreceptors, which primarily use CO2 as a signal. Peripheral blood sensors are found in the walls of the aorta and carotid arteries. These sensors signal the brain to provide immediate adjustments to the respiratory rate if CO2 levels rise or fall. Yet other sensors are found in the brain itself. Changes in the pH of CSF affect the respiratory center in the medulla oblongata, which can directly modulate breathing rate to bring the pH back into the normal range.
Hypercapnia, or abnormally elevated blood levels of CO2, occurs in any situation that impairs respiratory functions, including pneumonia and congestive heart failure. Reduced breathing (hypoventilation) due to drugs such as morphine, barbiturates, or ethanol (or even just holding one’s breath) can also result in hypercapnia. Hypocapnia, or abnormally low blood levels of CO2, occurs with any cause of hyperventilation that drives off the CO2, such as salicylate toxicity, elevated room temperatures, fever, or hysteria.
Renal Regulation of Acid-Base Balance
The renal regulation of the body’s acid-base balance addresses the metabolic component of the buffering system. Whereas the respiratory system (together with breathing centers in the brain) controls the blood levels of carbonic acid by controlling the exhalation of CO2, the renal system controls the blood levels of bicarbonate. A decrease of blood bicarbonate can result from the inhibition of carbonic anhydrase by certain diuretics or from excessive bicarbonate loss due to diarrhea. Blood bicarbonate levels are also typically lower in people who have Addison’s disease (chronic adrenal insufficiency), in which aldosterone levels are reduced, and in people who have renal damage, such as chronic nephritis. Finally, low bicarbonate blood levels can result from elevated levels of ketones (common in unmanaged diabetes mellitus), which bind bicarbonate in the filtrate and prevent its conservation.
Bicarbonate ions, HCO3–, found in the filtrate, are essential to the bicarbonate buffer system, yet the cells of the tubule are not permeable to bicarbonate ions. The steps involved in supplying bicarbonate ions to the system are seen in Figure 3 and are summarized below:
- Step 1: Sodium ions are reabsorbed from the filtrate in exchange for H+ by an antiport mechanism in the apical membranes of cells lining the renal tubule.
- Step 2: The cells produce bicarbonate ions that can be shunted to peritubular capillaries.
- Step 3: When CO2 is available, the reaction is driven to the formation of carbonic acid, which dissociates to form a bicarbonate ion and a hydrogen ion.
- Step 4: The bicarbonate ion passes into the peritubular capillaries and returns to the blood. The hydrogen ion is secreted into the filtrate, where it can become part of new water molecules and be reabsorbed as such, or removed in the urine.
Conservation of Bicarbonate in the Kidney. Tubular cells are not permeable to bicarbonate; thus, bicarbonate is conserved rather than reabsorbed. Steps 1 and 2 of bicarbonate conservation are indicated.It is also possible that salts in the filtrate, such as sulfates, phosphates, or ammonia, will capture hydrogen ions. If this occurs, the hydrogen ions will not be available to combine with bicarbonate ions and produce CO2. In such cases, bicarbonate ions are not conserved from the filtrate to the blood, which will also contribute to a pH imbalance and acidosis.
The hydrogen ions also compete with potassium to exchange with sodium in the renal tubules. If more potassium is present than normal, potassium, rather than the hydrogen ions, will be exchanged, and increased potassium enters the filtrate. When this occurs, fewer hydrogen ions in the filtrate participate in the conversion of bicarbonate into CO2 and less bicarbonate is conserved. If there is less potassium, more hydrogen ions enter the filtrate to be exchanged with sodium and more bicarbonate is conserved.
Chloride ions are important in neutralizing positive ion charges in the body. If chloride is lost, the body uses bicarbonate ions in place of the lost chloride ions. Thus, lost chloride results in an increased reabsorption of bicarbonate by the renal system.
Disorders of the…Acid-Base Balance: Ketoacidosis
Diabetic acidosis, or ketoacidosis, occurs most frequently in people with poorly controlled diabetes mellitus. When certain tissues in the body cannot get adequate amounts of glucose, they depend on the breakdown of fatty acids for energy. When acetyl groups break off the fatty acid chains, the acetyl groups then non-enzymatically combine to form ketone bodies, acetoacetic acid, beta-hydroxybutyric acid, and acetone, all of which increase the acidity of the blood. In this condition, the brain isn’t supplied with enough of its fuel—glucose—to produce all of the ATP it requires to function.
Ketoacidosis can be severe and, if not detected and treated properly, can lead to diabetic coma, which can be fatal. A common early symptom of ketoacidosis is deep, rapid breathing as the body attempts to drive off CO2 and compensate for the acidosis. Another common symptom is fruity-smelling breath, due to the exhalation of acetone. Other symptoms include dry skin and mouth, a flushed face, nausea, vomiting, and stomach pain. Treatment for diabetic coma is ingestion or injection of sugar; its prevention is the proper daily administration of insulin.
A person who is diabetic and uses insulin can initiate ketoacidosis if a dose of insulin is missed. Among people with type 2 diabetes, those of Hispanic and African-American descent are more likely to go into ketoacidosis than those of other ethnic backgrounds, although the reason for this is unknown.
Red blood cells (like all blood cells) form from hematopoietic stem cells in the bone marrow. As they mature, they lose their nucleus along with their DNA and many of their organelles.
The Vessels of the Circulatory System The 2 Main Types of Blood Vessels are
1) ARTERIES
2) VEINS
ARTERIES = Arteries are the blood vessels that are carrying blood AWAY FROM the heart. The arteries have high pressure and thick arterial walls.VEINS = Veins are the blood vessels that are carrying blood TOWARDS the heart. The veins have thin walls and low pressure since they are further away from the heart. For this reason, the veins have one-way valves that prevent the blood from flowing backward (see illustration).
Although most veins are shown in BLUE and most arteries are shown in RED, the color IS NOT an indication of whether or not it is an artery or a vein! Instead, the coloring means the following:
The RED coloring indicates that the blood carried in that vessel is OXYGENATED.
The BLUE coloring indicates that the blood carried in that vessel is DEOXYGENATED. The Pulmonary and Systemic Loops of the Circulatory System
The circulatory system circulates blood around two loops.
1) THE PULMONARY CIRCUIT - Functions to bring blood from the heart to the lungs and back to the heart
2) THE SYSTEMIC CIRCUIT - Functions to bring blood from the heart to all the parts of the body (except the lungs)
The Chambers of the Heart The heart has 4 chambers;
- The Right Atrium
- The Right Ventricle
- The Left Atrium
- The Left Ventricle
The word "atrium" comes from the Latin word for "entrance". The atria (plural for atrium) of the heart function to RECEIVE blood.
The right atrium of the heart is the chamber that receives deoxygenated blood from the body. The left atrium of the heart is the chamber that receives oxygenated blood from the lungs.
The word "ventricle" comes from the Latin word for "belly". This origin probably comes from the fact that the ventricles of the heart are the larger, more rounded chambers of the heart. The ventricles receive blood from the atria and the send that blood out of the heart. The ventricles create the "pumping" action of the heart by the rhythmic contraction and relaxation of the cardiac muscle (myocardium). The right ventricle receives the deoxygenated blood from the right atrium and sends that blood out to the lungs to pick up oxygen. The blood picks up oxygen and return to the heart at the left atrium. The left ventricle receives the oxygenated blood from the left atrium and sends that blood out to the body. The Flow of Blood Through the
Chambers of the Heart
The right atrium receives the deoxygenated blood that has traveled through the body. This blood has a low level of oxygen and a high level of carbon dioxide. This blood also has low pressure since it has traveled a good distance since it got its last "push" from the heart.
This deoxygenated blood from the lower and upper body travels to the right atrium, and then travels to the right ventricle. When the right ventricle contracts, it pushes the deoxygenated blood out of the heart and sends it to the lungs. (This is the pulmonary circuit). As this blood passes by the lungs, it picks up oxygen and gets rid of carbon dioxide.
The oxygenated blood from the lungs then travels back to the heart to get another "push" before it goes to the body (this is the systemic circuit). The left atrium receives the oxygenated blood coming from the lungs. This blood enters the left atrium and then travels to the left ventricle. The left ventricle contracts to push the oxygenated blood out of the heart and to the body in order to deliver oxygen and nutrients to the cells and to pick up unwanted substances like waste and carbon dioxide.
The Valves of the Heart
The four valves of the heart are as follows:
- The Tricuspid Valve is an atrioventricular (AV) valve that allows for the one-way movement of blood from the right atrium to the right ventricle.
- The Pulmonary Semilunar Valve is a semilunar (SV) valve that allows for the one-way movement of blood from the right ventricle to the pulmonary arteries (which travel to the lungs).
- The Mitral (Bicuspid) Valve is an atrioventricular (AV) valve that allows for the one-way movement of blood from the left atrium to the left ventricle.
- The Aortic Semilunar Valve is a semilunar (SV) valve that allows for the one-way movement of blood from the left ventricle to the aorta (which travel to the body).
The Heart Valves in the Anatomical Model of the Heart
The Flow of Blood Through the Heart INCLUDING THE VALVES
Deoxygenated blood comes from the body and goes to the right atrium. The blood then travels through the TRICUSPID VALVE to get to the right ventricle. Once the right ventricle contracts, this blood is then pumped through the PULMONARY SEMILUNAR VALVE to the pulmonary trunk which splits off to form the right and left pulmonary arteries which will carry the blood to the lungs.
Oxygenated blood comes from the lungs and enters the left atrium. The blood then travels through the MITRAL (BICUSPID) VALVE to the left ventricle. When the left ventricle contracts, the blood is pumped through the AORTIC SEMILUNAR VALVE to the aorta where it will travel to the body.
The Aorta
The aorta is the largest artery in the body. The function of the aorta is to send oxygenated blood to the body. That means all of the blood in the systemic circuit of the circulatory system has to go through the aorta.
The aorta has different regions. The first segment of the aorta ascends upward and is appropriately called the ascending aorta. The next segment makes a U-turn and forms and arch. This portion is call the aortic arch. The aortic arch has 3 vessels that project off of it superior. These 3 vessels collectively, function to bring oxygenated blood to the upper portions of the body.
After the aortic arch, the aorta turns inferiorly and descends as the descending aorta. The portion of the descending aorta hat gies through the thoracic cavity ids called the thoracic aorta. Once the the thoracic aorta travels through the diaphragm, it leave the thoracic cavity and enters into the abdominal cavity. This portion of the descending aorta is called the abdominal aorta.
The Branches of the Aortic ArchThe aortic arch contains 3 branches
- the brachiocephalic trunk
- the left common carotid artery
- the left subclavian artery
The Brachiocephalic Trunk, Left Common Carotid Artery and Left Subclavian Arteries in the Anatomical Model of the Torso
The 3 branches of the aortic arch function, collectively, to carry oxygenated blood to the upper regions of the body. The first branch is the brachiocephalic trunk. The brachiocephalic trunk bifurcates (splits into 2) to form the right subclavian artery and the right common carotid artery. The right subclavian artery supplies blood to the right arm and the right common carotid artery will continue to travel superiorly and supply blood to the right side of the head, neck and brain.
The second branch is the left common carotid artery and it supplies blood to the left side of the head, neck and brain. The third branch is the left subclavian artery and it supplies blood to the left arm.
The descending aorta supplies blood to the lower portions of the body.
The Brachiocephalic Trunk, Left Common Carotid Artery and Left Subclavian Arteries in the Anatomical Model of the HeartThe Pulmonary Trunk and Pulmonary Arteries Another large artery you see in the heart is colored BLUE because it is carrying deoxygenated blood from the right ventricle to the lungs. This artery is called the pulmonary trunk. The pulmonary trunk branches off to form the right and left pulmonary arteries. The right pulmonary artery carries the blood to the right lung and the left pulmonary artery carries blood to the left lung.
The Brachiocephalic Veins The right and left brachiocephalic veins come together to form the superior vena cava. The word "brachio-" means "upper arm" and the word "cephalic" means "head". The right brachiocephalic vein brings deoxygenated blood from the right side of the head and the right arm, to the superior vena cava. The left brachiocephalic vein brings deoxygenated blood from the left side of the head and neck and the left arm, to the superior vena cava
The Pulmonary Veins Oxygenated blood coming from the lungs returns to the heart through the right and left pulmonary veins. The veins are colored RED, because these veins carry oxygenated blood. The right pulmonary veins bring oxygenated blood from the right lung to the left atrium of the heart. The left pulmonary veins bring oxygenated blood from the left lung to the left atrium of the heart.
The Superior and Inferior Vena Cava The largest veins of the body are the superior and inferior vena cava. The superior vena cava brings deoxygenated blood from the upper body to the right atrium. The inferior vena cava brings deoxygenated blood from the lower body to the right atrium.
The white blood cells retain their nuclei as can be seen with the purple hematoxylin nuclear stain. The white blood cells function in different ways to protect you from infection and disease.
The platelets appear as small darkly stained fragments on the slide. These are blood clotting factors.
The platelets appear as small darkly stained fragments on the slide. These are blood clotting factors.
THE WHITE BLOOD CELLS (LEUKOCYTES)
White blood cells help to protect your body against infection and fight disease. Unlike the red blood cells, white blood cells DO have a nucleus.
A Visual Animation of How the Blood Flows Through the Chambers of the Heart and the Pulmonary and Systemic Circuits of the Circulatory System