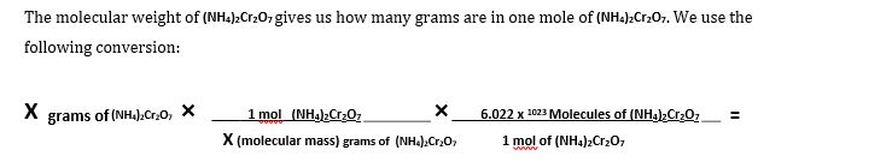Chemistry Corner
Please make sure to perform any labs under the supervision of a knowledgeable adult and use all safety precautions and proper waste disposal! Be safe and have fun!
Ammonium Dichromate Volcano Lab
Volcano Project
MUST USE CHEMICAL HOOD AND PROFESSIONAL SUPERVISION FROM A KNOWLEDGEABLE INSTRUCTOR!
Oxidation‐reduction (aka REDOX) reactions are some of the most important chemical reactions. They produce energy by the passing of one or more electrons from one species to another.
DIRECTIONS:
GROUP ONE
– The decomposition reaction of (NH4)2Cr2O7 (solid) -> Cr2O3 (solid) + N2 (gas) + 4H2O (gas)
Instructions:
Make a “volcano” out of aluminum cans and aluminum foil.
Measure out 100 grams of ammonium dichromate in a weigh boat.
Pour a cone of the ammonium dichromate in the middle of the foil tray.
Insert a 6-inch length of Visco-fuse into the middle of the pile of orange crystals.
Observations:
What color(s) did you see if the flame? ________________________________
What color(s) did you observe in the ash? ______________________________
What did the lava look like? ________________________________________
GROUP TWO
– The combustion reaction of 2C + O2 -> 2CO and C + O2 -> CO2
Instructions:
Make a “volcano” out of aluminum cans and aluminum foil.
Measure out 75 grams of ammonium dichromate in a weigh boat.
Add 25 grams of 36-mesh charcoal to the ammonium chromate.
Mix well with stick without making contact with substance or spilling substance.
Pour a cone of the mixture in the middle of the foil tray.
Insert a 6-inch length of Visco-fuse into the middle of the pile of orange crystals.
Observations:
What color(s) did you see if the flame? ________________________________
What color(s) did you observe in the ash? ______________________________
What did the lava look like? ________________________________________
GROUP THREE
– The combustion reaction of 4Al + 3O2 → 2Al2O3
Instructions:
Make a “volcano” out of aluminum cans and aluminum foil.
Measure out 75 grams of ammonium dichromate in a weigh boat.
Add 25 grams of medium flake aluminum to the ammonium chromate.
Mix well with stick without making contact with substance or spilling substance.
Pour a cone of the mixture in the middle of the foil tray.
Insert a 6-inch length of Visco-fuse into the middle of the pile of orange crystals.

OXIDIZED – lost electron(s) REDUCED – stole electron(s)
Ammonium dichromate's formula is (NH4)2Cr2O7.
When it burns, it decomposes according to the following equation:
(NH4)2Cr2O7 (solid) -> Cr2O3 (solid) + N2 (gas) + 4 H2O (gas)
If you burn aluminum in oxygen you will form aluminum oxide. The chemical formula for aluminum oxide is: Al2O3.
The balanced chemical equation is
4 Al + 3 O2 → 2 Al2O3
Charcoal is mostly carbon (C). When it burns, two reactions will possibly happen. One is an incomplete combustion reaction, in which carbon monoxide is formed.
2 C + O2 -> 2 CO
This is complete combustion and when burning with an excess of oxygen in an open space is the more likely reaction. Both reactions will probably happen, but it is likely more CO2 than CO will be formed.
C + O2 -> CO2
Ammonium dichromate's formula is (NH4)2Cr2O7.
When it burns, it decomposes according to the following equation:
(NH4)2Cr2O7 (solid) -> Cr2O3 (solid) + N2 (gas) + 4 H2O (gas)
If you burn aluminum in oxygen you will form aluminum oxide. The chemical formula for aluminum oxide is: Al2O3.
The balanced chemical equation is
4 Al + 3 O2 → 2 Al2O3
Charcoal is mostly carbon (C). When it burns, two reactions will possibly happen. One is an incomplete combustion reaction, in which carbon monoxide is formed.
2 C + O2 -> 2 CO
This is complete combustion and when burning with an excess of oxygen in an open space is the more likely reaction. Both reactions will probably happen, but it is likely more CO2 than CO will be formed.
C + O2 -> CO2
Observations:
- What color(s) did you see if the flame?
- What color(s) did you observe in the ash?
- What did the "lava" look like?
- What is the chemical formula or ammonium?
- What is the chemical formula for dichromate?
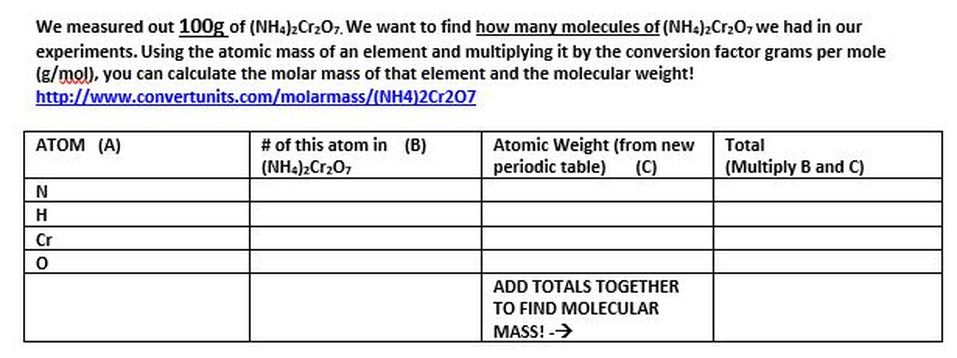
- How many nitrogen atoms are in ammonium dichromate?
- How many hydrogen atoms are in ammonium dichromate?
- How many chromium atoms are in ammonium dichromate?
- How many oxygen atoms are in ammonium dichromate?
- How many atoms altogether are there is ammonium dichromate?
Post-Lab Questions
Everything that goes into a reaction MUST come out the other side. Chemical equations show chemical reactions as reactants products.
- Write the full chemical equation for the reaction from group one.
- Write the full chemical equation for the reaction from group two.
- Write the full chemical equation for the reaction from group three.
More Help
MASS <---> MOLES <---> ATOMS
Additional Questions
1. Calculate the number of moles in 31.4 g of Fe. (HINT: The nice thing about the timeline is that we can see where we are starting (step #1), see where we are going (step #2), and find the appropriate conversion factors to get from step #1 to step #2 (step #3))
2. Calculate the number of moles in 5.0 x 108 atoms of 14C. (HINT: So, if we look at our timeline, we are starting on the right-hand side (atoms) and we want to get to the middle (moles). The conversion factor that allows us to do this is Avogadro's Number.)
3. Calculate the mass (in grams) of 1.0 x 10^32 molecules of water, H2O. (HINT: if we look at our timeline, we are starting on the right-hand side (molecules) and we want to get to the left-hand side (mass). Now, we will need two conversion factors. The first conversion factor, Avogadro's Number, will convert molecules to moles. Then we need the conversion factor between moles and mass which is molar mass.