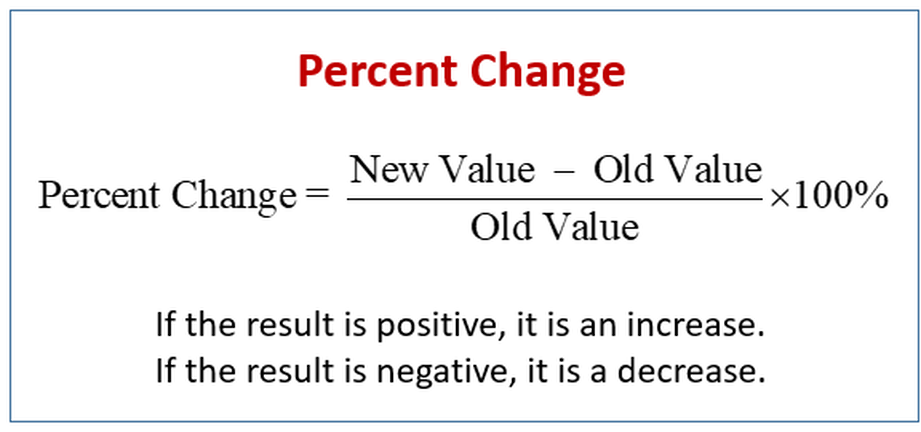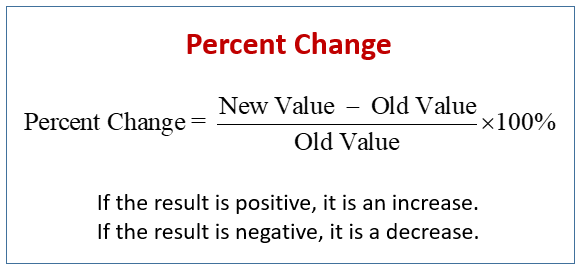Chemistry and Conversions Lab
Determining solute concentration, tonicity, and solubility.
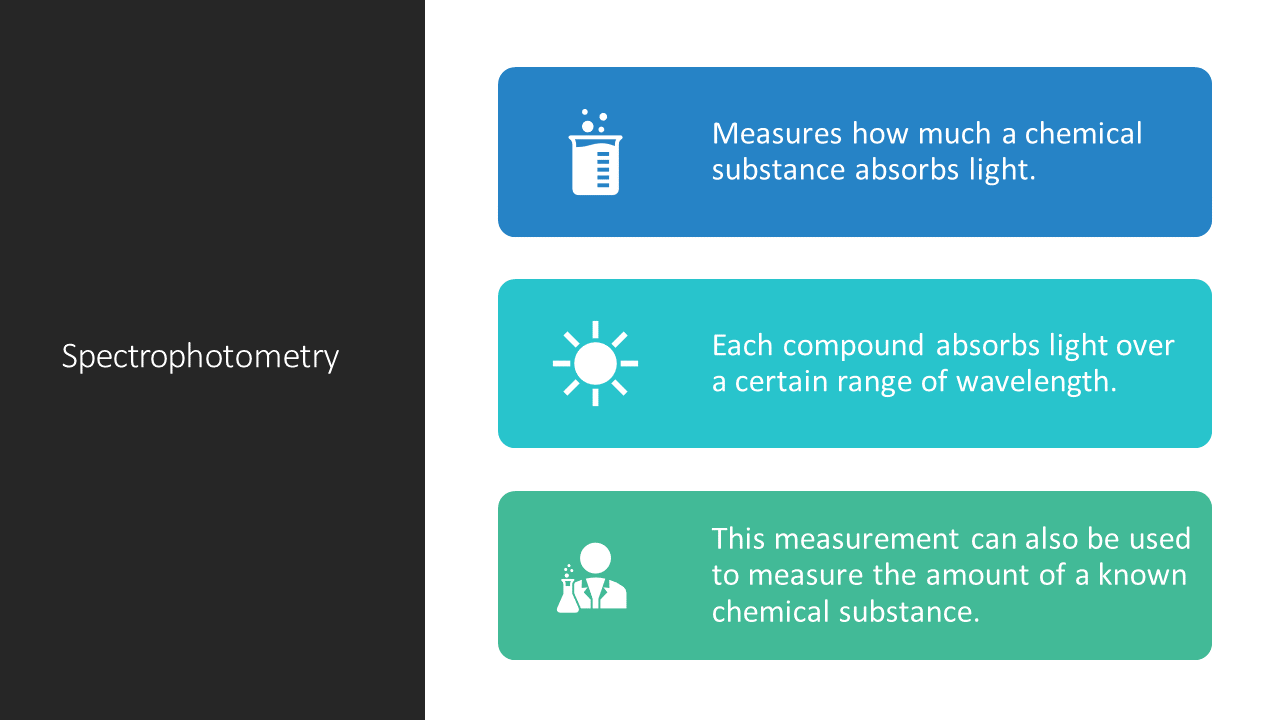
SPECTROPHOTOMETRY
|
Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The basic principle is that each compound absorbs or transmits light over a certain range of wavelength. This measurement can also be used to measure the amount of a known chemical substance. Spectrophotometry is one of the most useful methods of quantitative analysis in various fields such as chemistry, physics, biochemistry, material and chemical engineering and clinical applications.
|
A spectrophotometer is an instrument that measures the amount of photons (the intensity of light) absorbed after it passes through sample solution. With the spectrophotometer, the amount of a known chemical substance (concentrations) can also be determined by measuring the intensity of light detected.
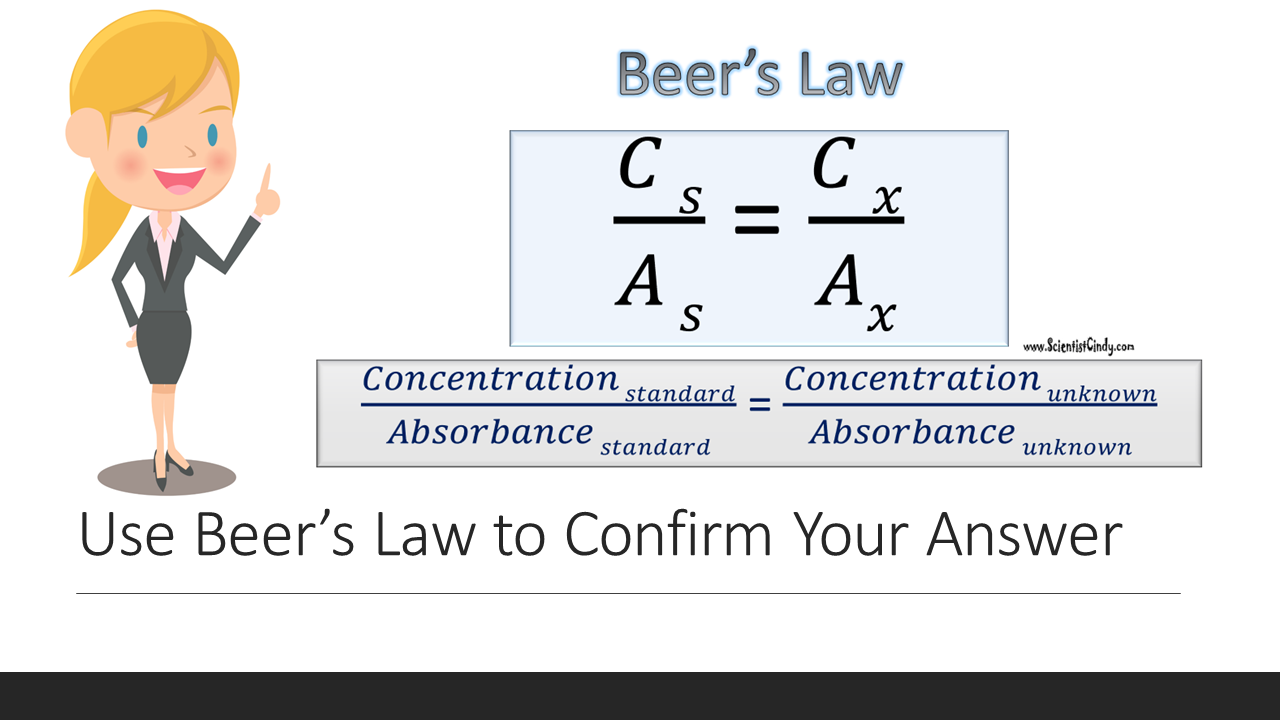
Lab Exercises: A. Beer’s Law (Cs/As = Cx/Ax): Calculating the glucose concentration of an unknown solution
DIRECTIONS:
DIRECTIONS:
1. Turn on your spectrophotometer to allow it to warm up.
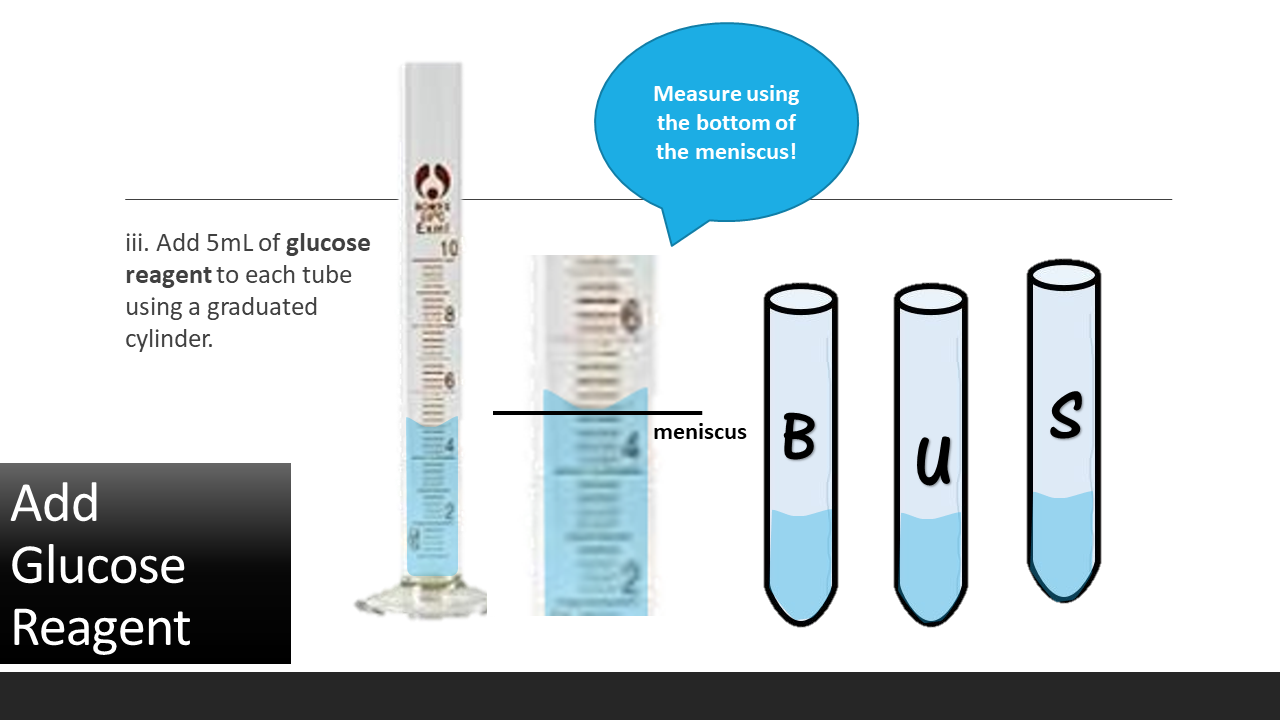
2. Obtain 3 test tubes and label them with a wax pen a. B for blank b. U for unknown glucose solution c. S for standard
2. Obtain 3 test tubes and label them with a wax pen a. B for blank b. U for unknown glucose solution c. S for standard
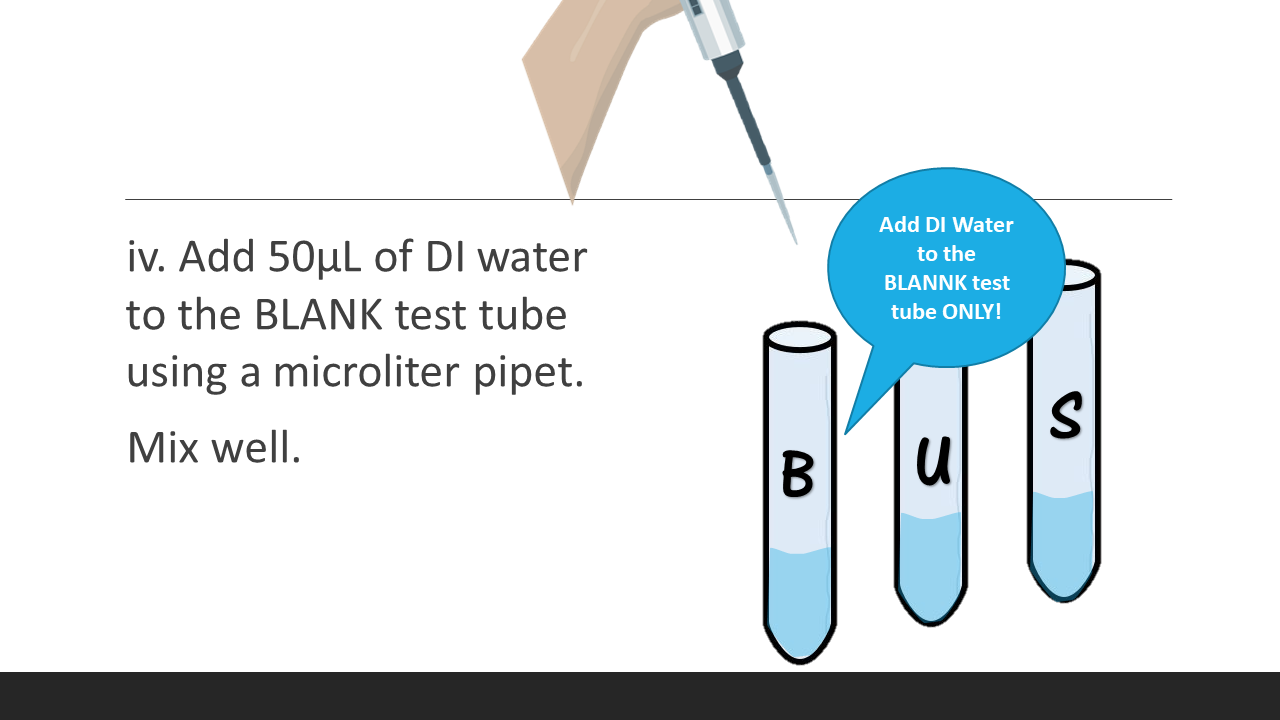
4. Add 50μL of DI water to the BLANK test tube using a microliter pipet. Mix well.
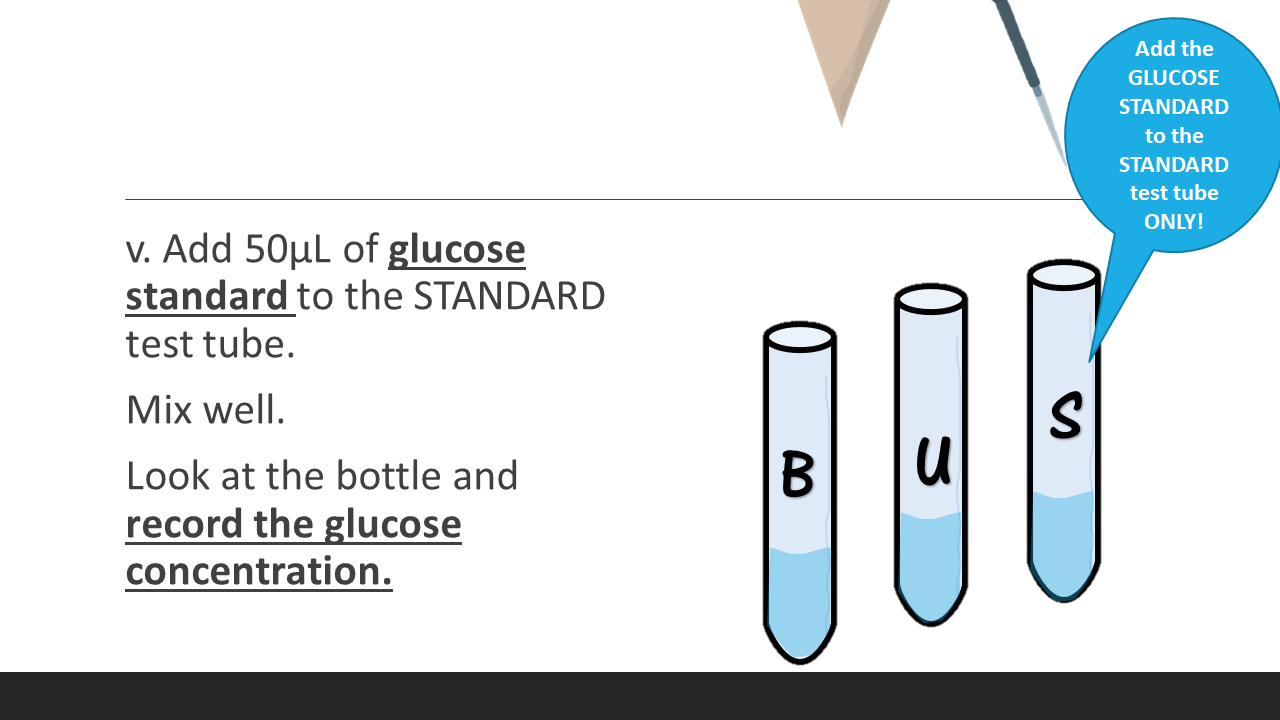
5. Add 50μL of glucose standard to the STANDARD test tube. Mix well. Look at the bottle and record the glucose concentration.
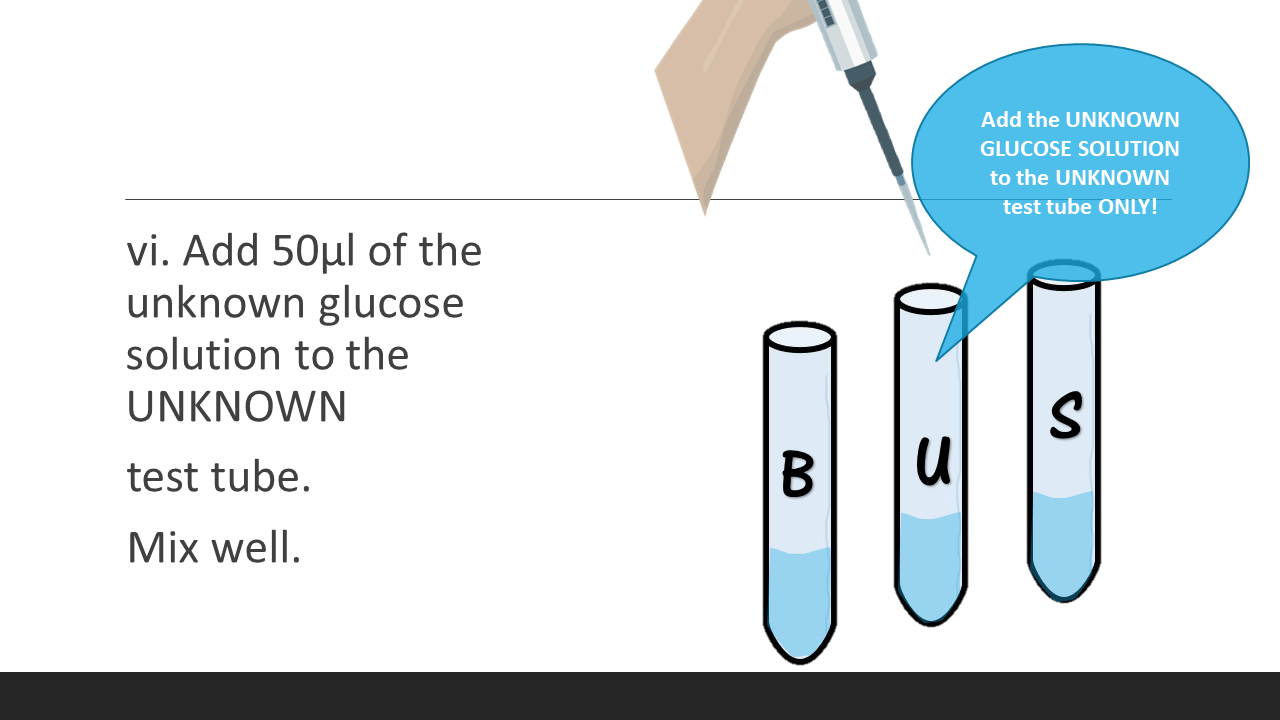
6. Add 50μl of the unknown glucose solution to the UNKNOWN test tube. Mix well.
5. Add 50μL of glucose standard to the STANDARD test tube. Mix well. Look at the bottle and record the glucose concentration.
6. Add 50μl of the unknown glucose solution to the UNKNOWN test tube. Mix well.
7. Incubate each test tube for 20min in the 37 ºC water bath .
Label 3 cuvettes (B, U, S) as you are waiting.

8. After the incubation period, pour the solutions into their proper cuvette.
9. Set the wavelength of the spectrophotometer to 500nm.
10. Blank the spectrophotometer. Don’t forget to mix the solution before you measure the absorbance.
11. Measure the absorbance of the standard and unknown solutions. Don’t forget to mix the solutions before measuring the absorbance.

12. Using Beer’s Law, calculate the concentration of the unknown glucose solution.
13. Clean-up: Dispose of all liquids in the chemical waste container near a sink. Place the test tubes in the test tube collection bucket. Wash the cuvettes (do not scratch) and place them upside down in the test tube rack to dry (wash with soap and water, then rinse with DI water).
LAB Exercise B. Standard Curve: Determining the albumin (protein) concentration of an unknown solution
|
Protocol
|
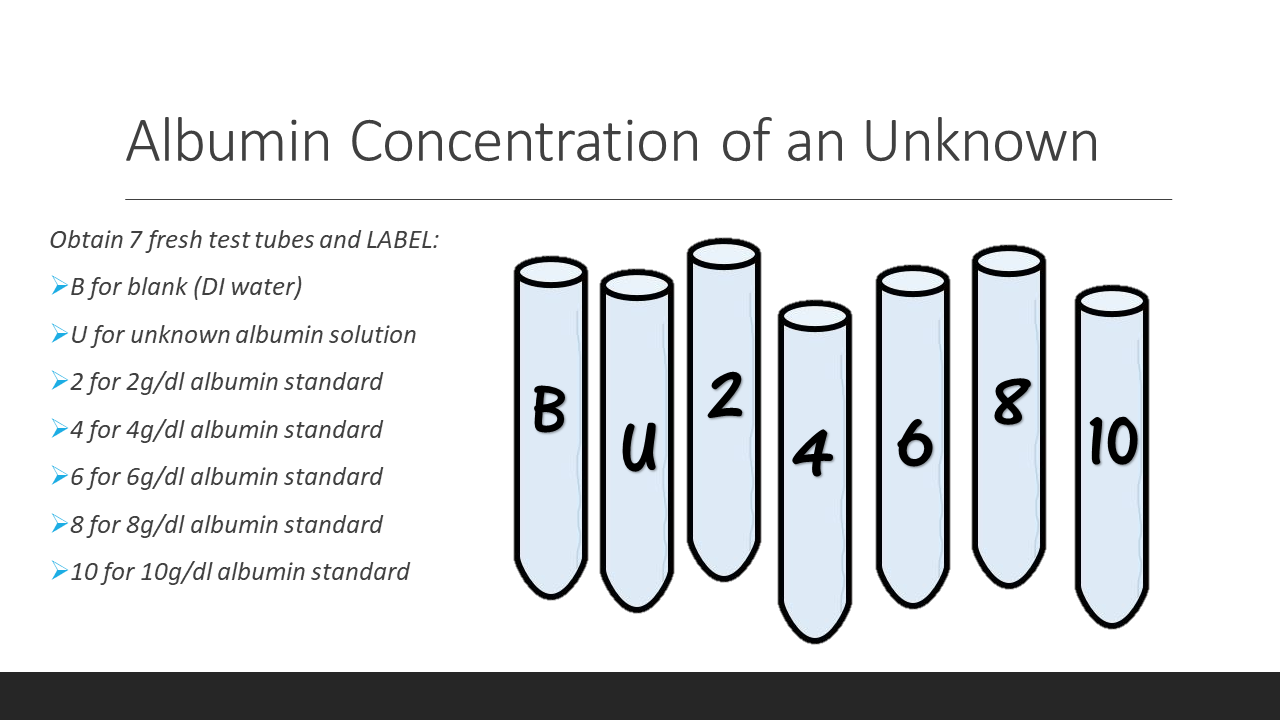
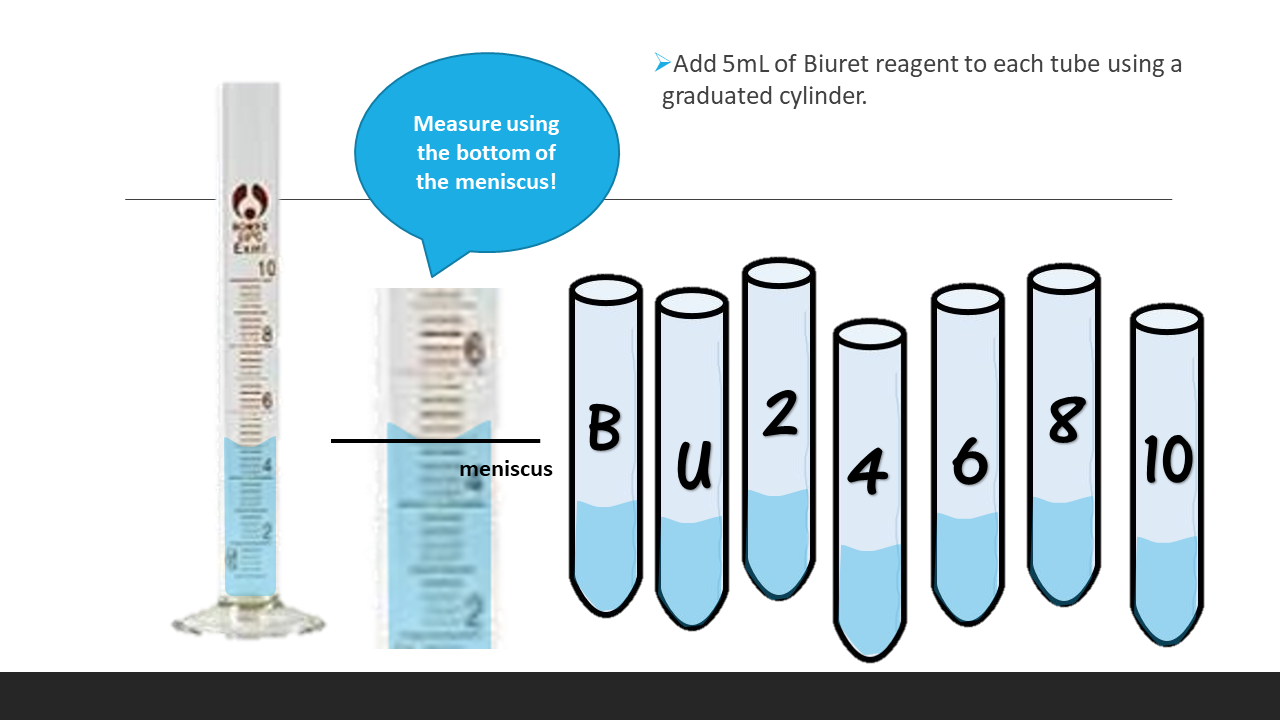
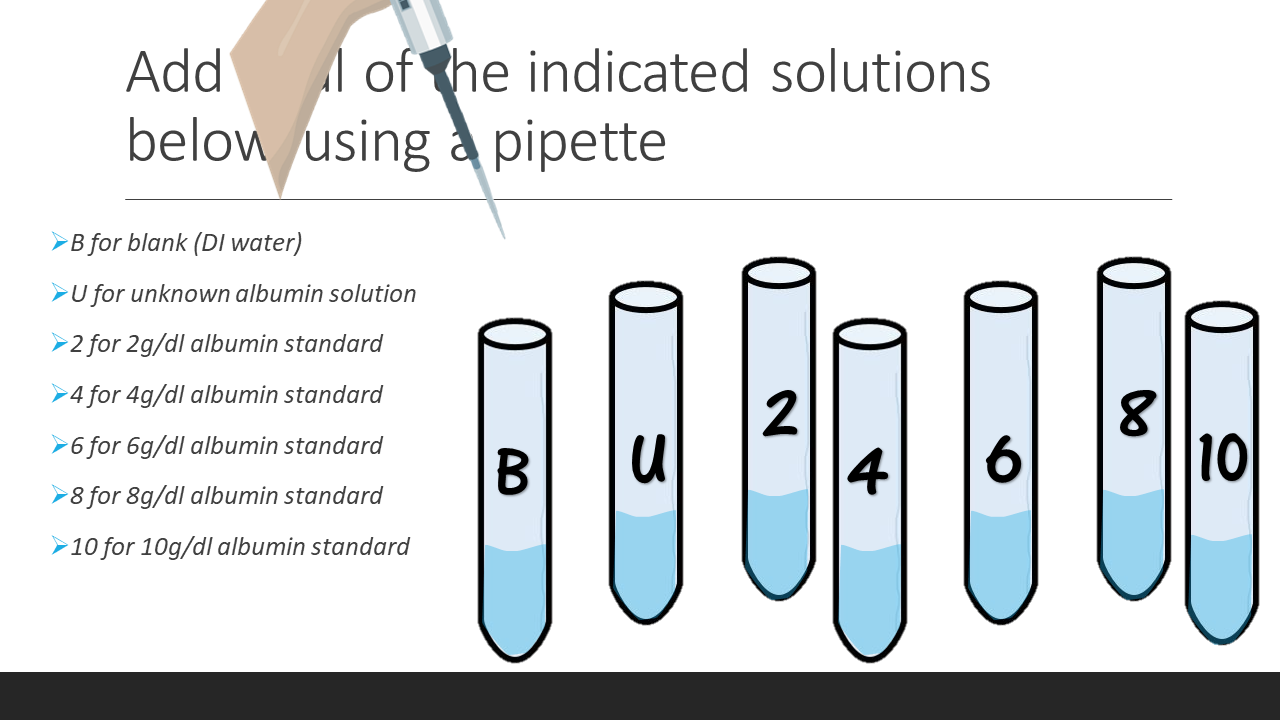
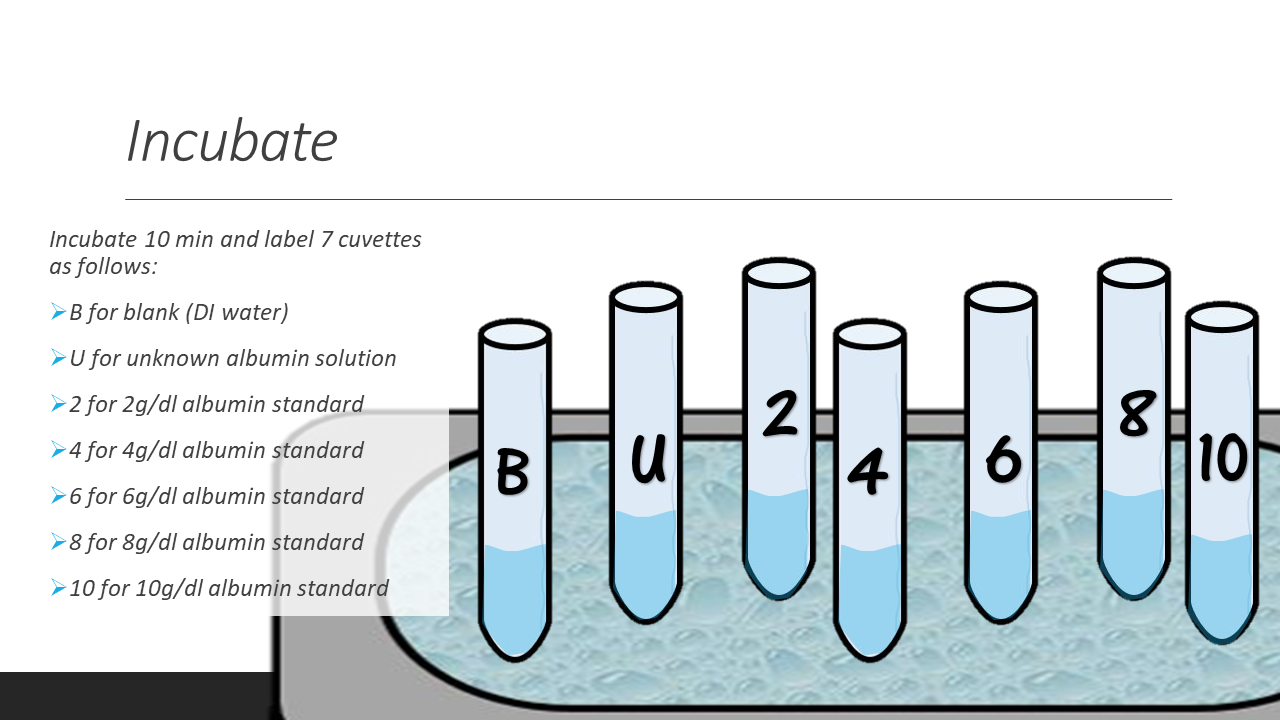
- 6. Incubate each test tube for 10min at room temperature. Label 7 cuvettes (B, U, 2, 4, 6, 8, 10) as you are waiting.
- 7. After the incubation period, pour the solutions into their proper cuvette.
- 8. Set the wavelength of the spectrophotometer to 550nm.
- 9. Blank the spectrophotometer. Don’t forget to mix the solution before you measure the absorbance.
- 10. Measure the absorbance of the standards and unknown solutions. Don’t forget to mix the solutions before measuring the absorbance.
- 11. Create a standard curve and determine the albumin concentration of the unknown.
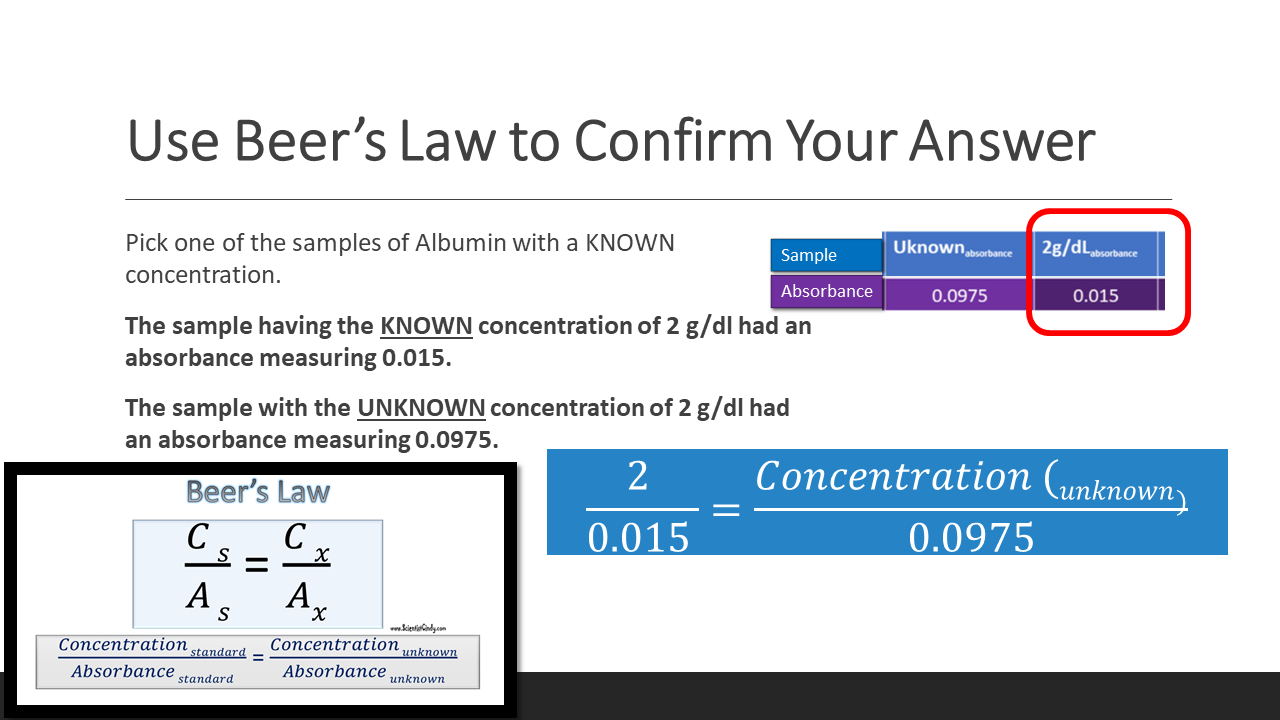
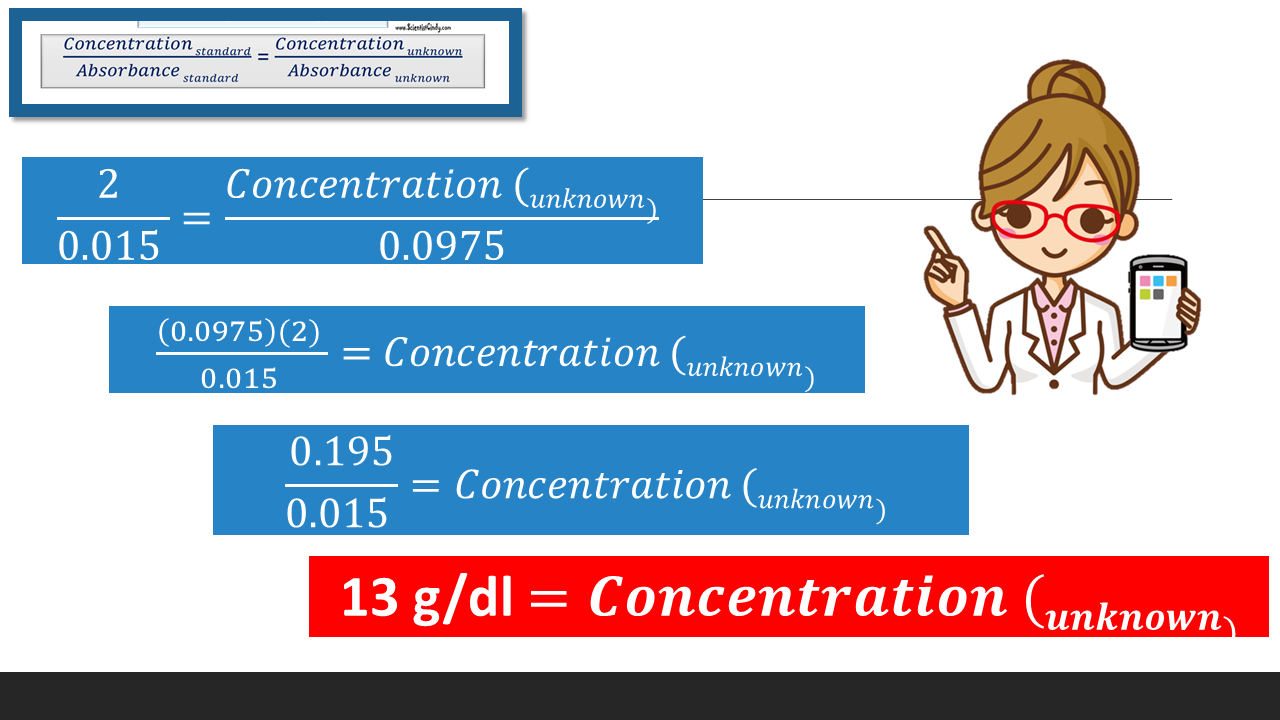
Standards are used in biology that have known values. We can use a standard that has a known concentration to determine the concentration of an unknown sample. This is done using Beer's law, which simply states that the concentration of any substance is directly proportional to its absorbance. This means that we can compare the ratio of CONCENTRATION to ABSORBANCE (C/A or C:A) of a known standard to the ratio ratio of CONCENTRATION to ABSORBANCE (C/A or C:A) of an UNKNOWN sample, using Beer’s Law Beer’s law (Cs/As = Cx/Ax).
HOW TO create a standard curve and determine the albumin concentration of the unknown.
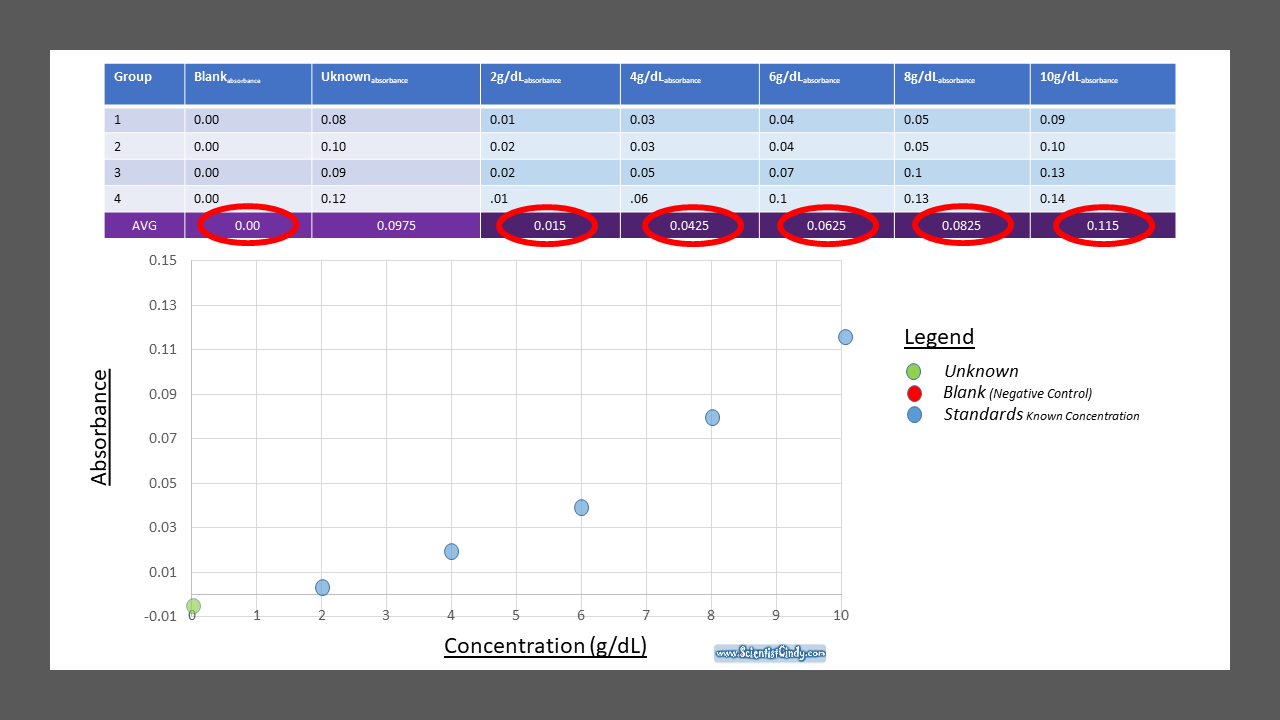
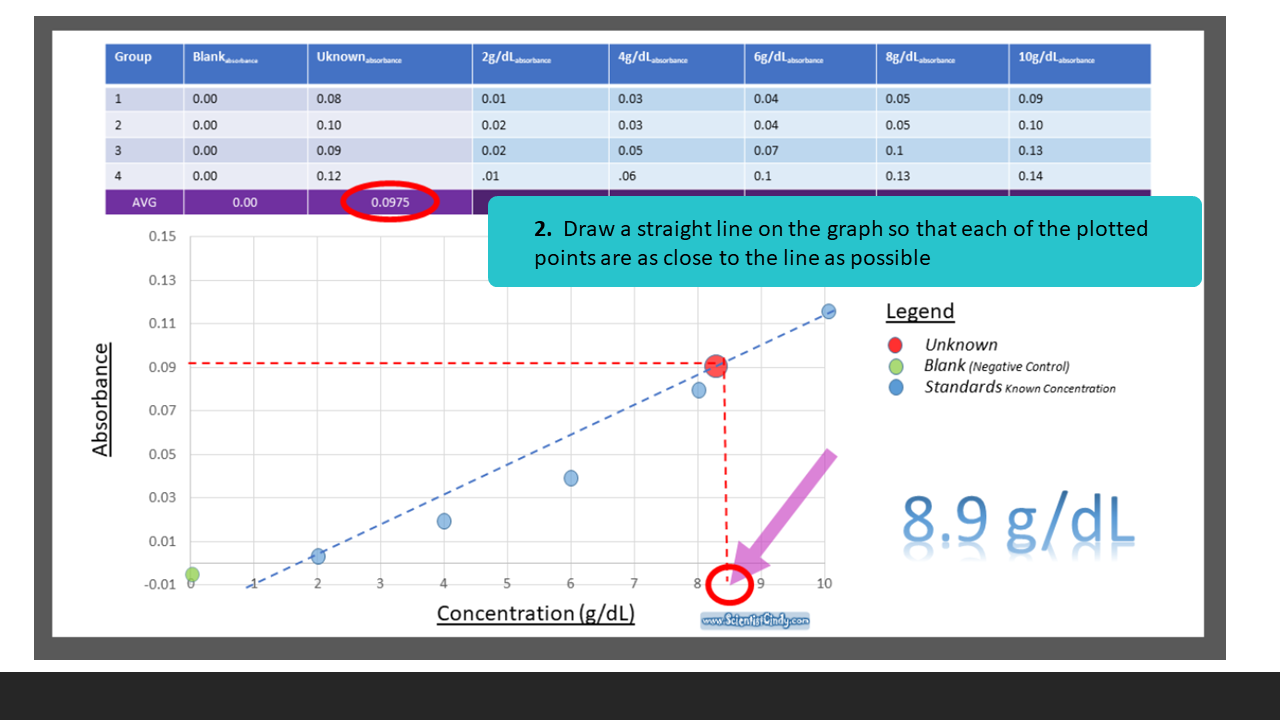
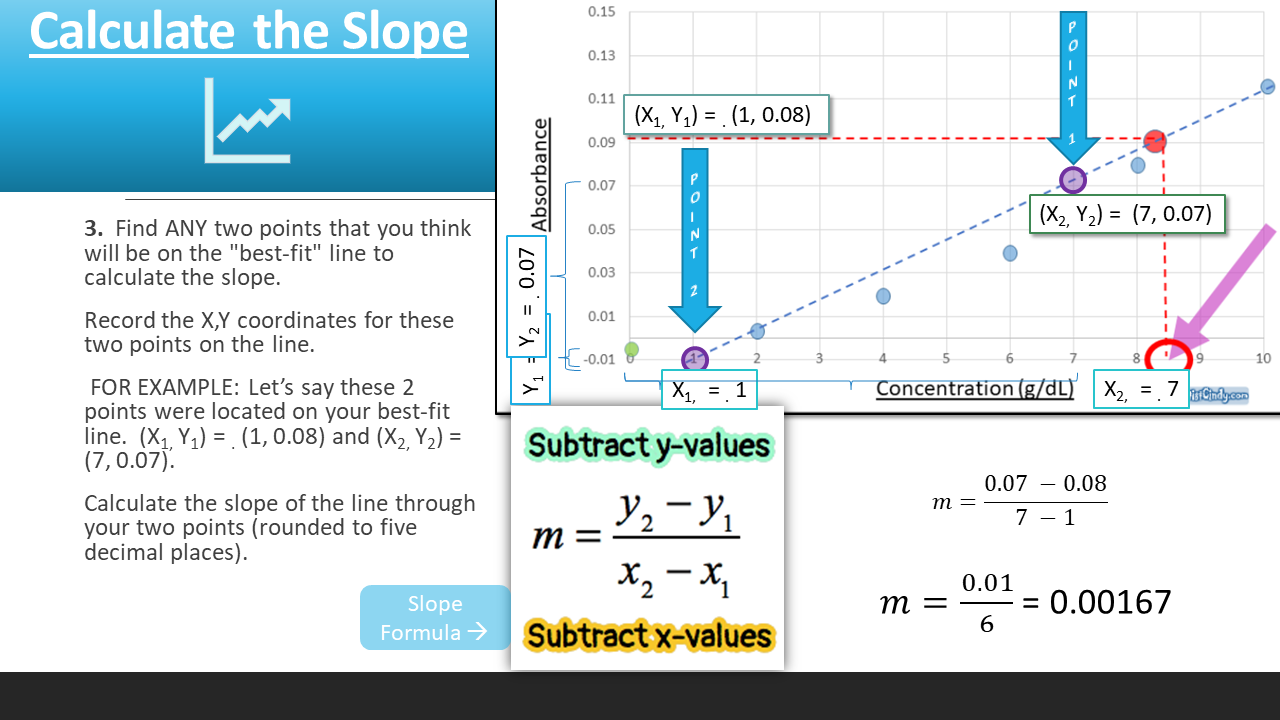
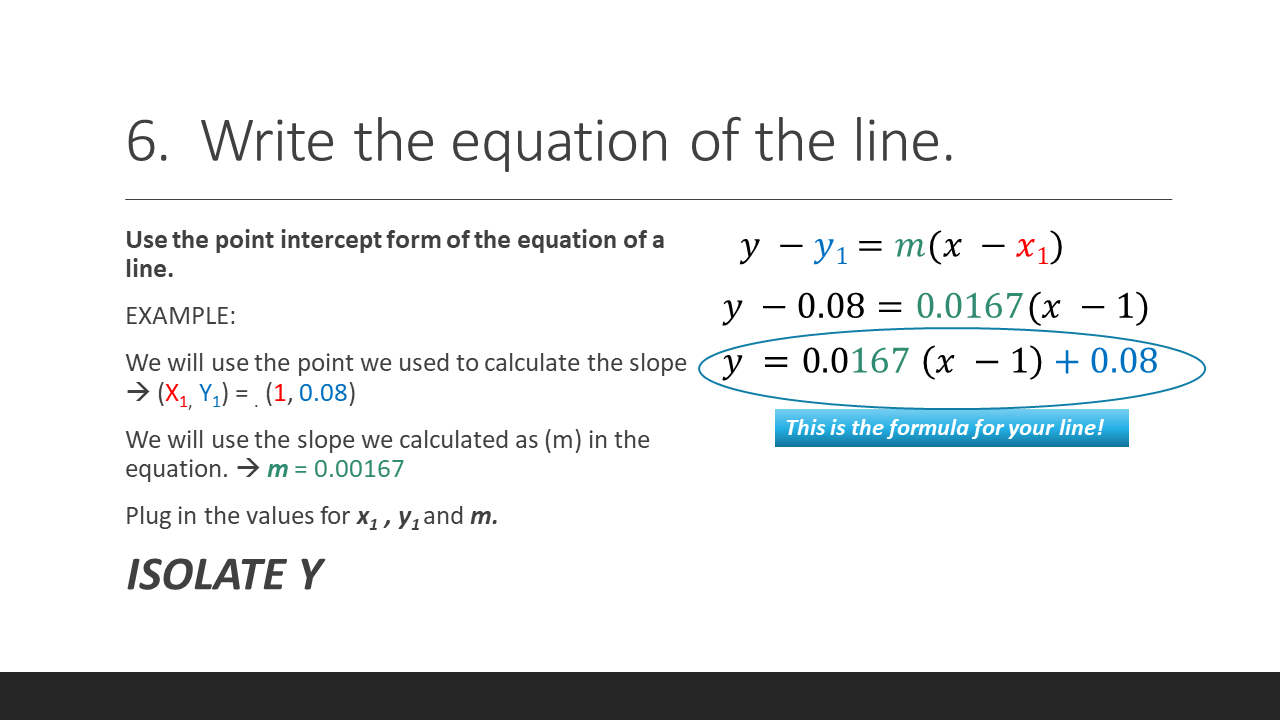
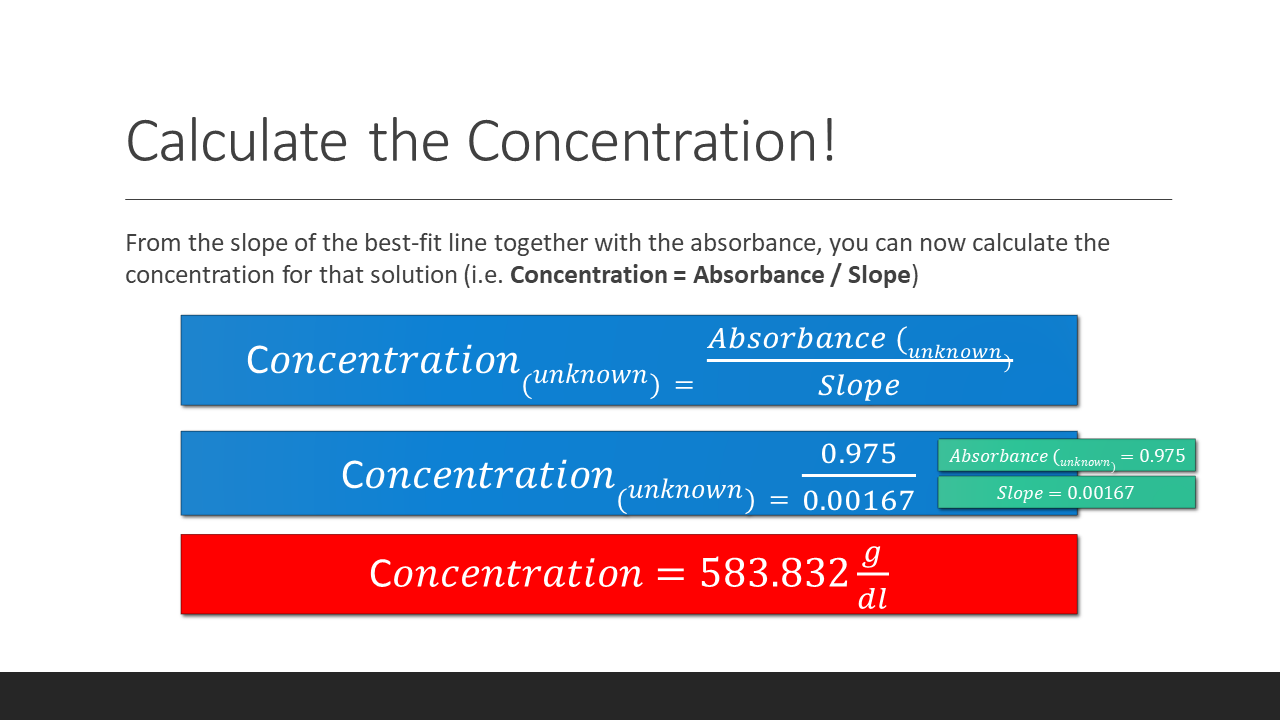
To find the concentration for your UNKNOWN solution that has an absorbance of 0.975, you will first need to find the slope of the BEST-FIT line. From the slope of the best-fit line together with the absorbance, you can now calculate the concentration for that solution (i.e. Concentration = Absorbance / Slope)
Creating a Standard Curve
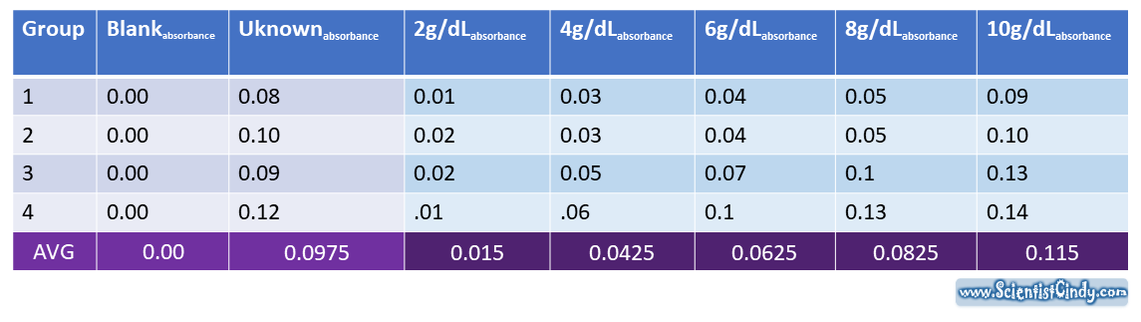
STEP ONE: Measure the Absorbance of Your Blank (0g/dL), a Set of Standards and Your UNKNOWN.
Create a Table of Your Data.
Here is some sample data that you can use, or you may use data that your group collected.
How to Find the Equation of Your Standard Curve
Beer's Law is the equation that relates the absorbance of a substance directly to the concentration of that substance. A solution that has a higher concentration, will have a higher amount of dissolved substances. These dissolved substances block the light from the spectrophotometer from going through the substance. So, the higher the concentration, the higher the absorbance. We can create a STANDARD CURVE, by measuring the absorbance of KNOWN solutions.
Determining the albumin (protein) concentration of an unknown solution

● Biuret reagent results:
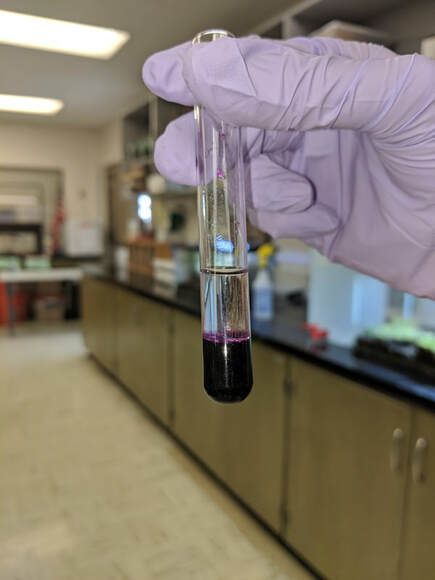
The Biuret reagent used to test for protein (albumin) is blue in color.
The Biuret reagent used to test for protein (albumin) is blue in color.
- If there is no change in color (solution remains blue) then protein (albumin) is not present.
- If there is a change in color from blue to violet (deep purple) then protein (albumin) is present.
- If there is a change in color from blue to pink then protein (albumin) is present.
OSMOSIS - Dialysis Bag Experiment
LAB Exercise C. Diffusion, Osmosis, and Tonicity
Equipment •
Equipment •
- Dialysis tubing
- 10% sucrose solution •
- 30% sucrose solution •
- Balance •
- 500mL beaker •
- Dialysis tube clips
Protocol
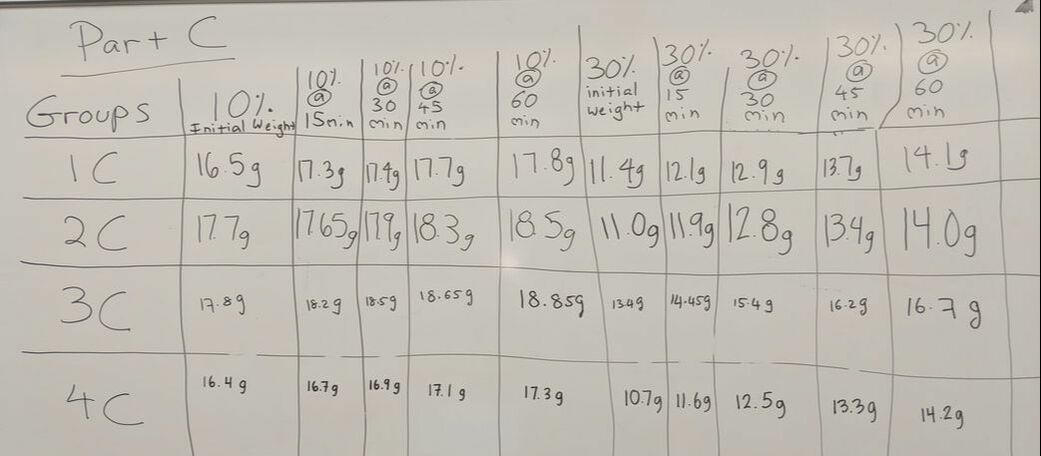
- 1. Blot the 10% sucrose dialysis bag and use the balance to determine the INITIAL WEIGHT of the dialysis bag.
- 2. Blot the 30% sucrose dialysis bag and use the balance to determine the INITIAL WEIGHT of the dialysis bag.
- 3. Label the 500mL beakers using a wax pen.
- Label one 500mL beaker as "10" for 10% sucrose
- Label the other 500mL beaker as "30" for 30% sucrose
- 4. Add 250mL of tap water to each beaker.
- 5. Add the dialysis bags to their appropriate beaker and allow them to sit for 15min. Use a timer.
- 6. Remove the dialysis bag after the timer alerts you.
- 7. Blot the dialysis bags and use the balance to determine the weight of the dialysis bag.
- 8. Repeat steps 5, 6, & 7 three more times (total of 60min).
- 9. Draw a line graph to show your data.
- 10. Clean-up: Wash and dry the beakers. Dispose of the sucrose solutions in the sink. Wash and dry the dialysis clips and return them to your table. Dispose of the dialysis bag in the regular trash. Clean up any sucrose solution spills/drips (look like shiny drops on your desk).

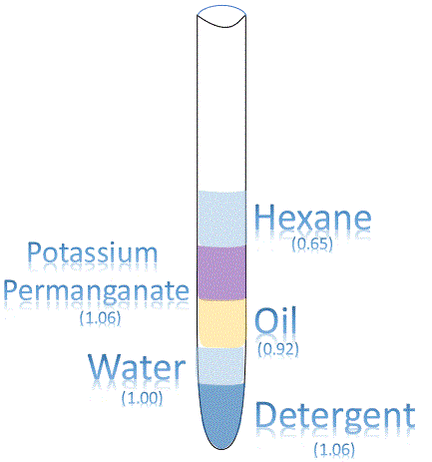
Solubility
Have you ever heard that oil and water don't mix? This sentence is describing the fact that oil/fat is INSOLUBLE in water. We can test the SOLUBILITY of different substances by mixing them together and seeing if they create layers or not. If layers are created, or if a precipitate appears, then the substance is INSOLUBLE or, at least, partially INSOLUBLE. If the substances mix together and you cannot tell one substance from the other, then the substance must be SOLUBLE.
Lab Exercise D. Solubility
1. Obtain a test tube.
2. Add 2mL of water using a graduated cylinder. Draw your result.
3. Add 2mL of hexane using a serological pipet under a fume hood. Mix well. Draw your result and label each layer.
4. Add 1 crystal of potassium permanganate (KMnO4). Mix well. Draw your result and label each layer.
5. Add 1mL of vegetable oil. Mix well. Draw your result and label each layer.
6. Add 1mL of detergent. Mix well. Draw your result and label each layer.
7. In detail explain what you saw at each step. Refer to the terms that you have learned (i.e. solubility, nonpolar, polar, amphipathic, micelles, etc.).
8. Clean-up: Dispose of all liquids in the Chemical Waste Container near a sink. Place the test tubes in the collection bucket.
1. Obtain a test tube.
2. Add 2mL of water using a graduated cylinder. Draw your result.
3. Add 2mL of hexane using a serological pipet under a fume hood. Mix well. Draw your result and label each layer.
4. Add 1 crystal of potassium permanganate (KMnO4). Mix well. Draw your result and label each layer.
5. Add 1mL of vegetable oil. Mix well. Draw your result and label each layer.
6. Add 1mL of detergent. Mix well. Draw your result and label each layer.
7. In detail explain what you saw at each step. Refer to the terms that you have learned (i.e. solubility, nonpolar, polar, amphipathic, micelles, etc.).
8. Clean-up: Dispose of all liquids in the Chemical Waste Container near a sink. Place the test tubes in the collection bucket.
Hexane + Water + Potassium PermanganateBecause of the random movement of potassium permanganate particles, a dense purple solution forms in water at base of the beaker. The purple solution will slowly spread into the rest of the water throughout the beaker creating a less dense but evenly colored purple solution
Potassium permanganate dissolves into the water (BOTTOM LAYER), but does not dissolve in the hexane (TOP LAYER). Therefore, hexane is SOLUBLE in water. |
|
|
|
Detergents work as surfactants they tend to concentrate on the surface of water. They cling to the surface because they try to orient their polar CO2- heads toward water molecules and their nonpolar tails away from neighboring water molecules. If you shake or invert the test tube with detergent, the detergent will break up the other substances and force them into solution.
|
UNDERSTANDING AND CALCULATING CONCENTRATIONS
Osmosis, Diffusion and Equilibrium
Osmosis
Dialysis Bag Experiment
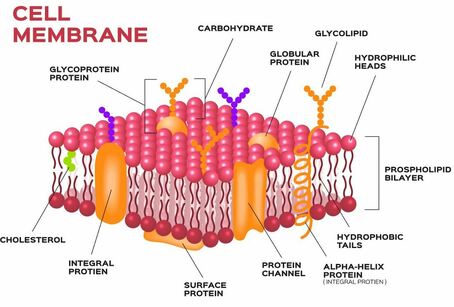
Living cells need to obtain nutrients from their environment and get rid of waste materials to their surroundings. This exchange of materials between the cell and its surroundings is crucial to its existence. Cells have membranes composed of a phospholipid bilayer embedded with proteins. This cell membrane can distinguish between different substances, slowing or hindering the movement of other substances and allowing others to pass through readily. This property of the cell is known as selective permeability.
Selective permeability is a property of a cell membrane that allows it to control which molecules can pass (moving into and out of the cell) through the pores of the membrane. Selective permeable membranes only allows small molecules such as glucose, amino acids to readily pass through, and inhibits larger molecules like protein, starch, from passing through it.
Selective permeability is a property of a cell membrane that allows it to control which molecules can pass (moving into and out of the cell) through the pores of the membrane. Selective permeable membranes only allows small molecules such as glucose, amino acids to readily pass through, and inhibits larger molecules like protein, starch, from passing through it.
|
|
|
The dialysis tubing is a semi-permeable membrane tubing used in separation techniques and demonstration of diffusion, osmosis, and movement of molecules across a restrictive membrane. It separates dissolved substances of different molecular sizes in a solution, and some of the substances may readily pass through the pores of the membrane while others are excluded.
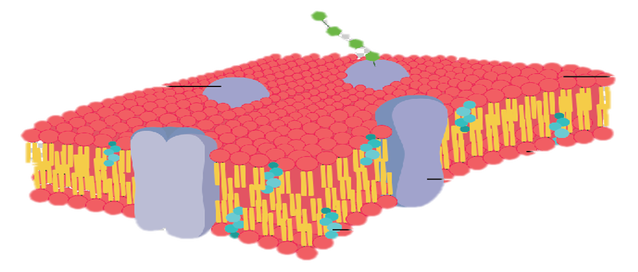
- Dialysis bags can act as a model of the semipermeable membrane of the cell. The surface of the dialysis bags are made of an artificial membrane composed of cellulose that has microscopic pores in it.
- When the dialysis bags are placed into a beaker containing liquid, the liquid in the beaker is a model of the extracellular fluid (ECF).
- Formula: (Final weight (g) - Initial weight (g))/Initial weight (g) x 100
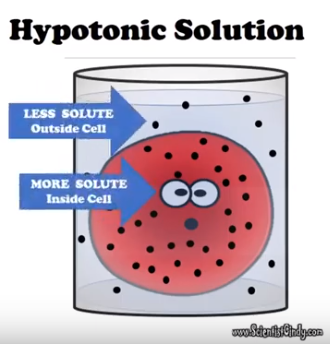
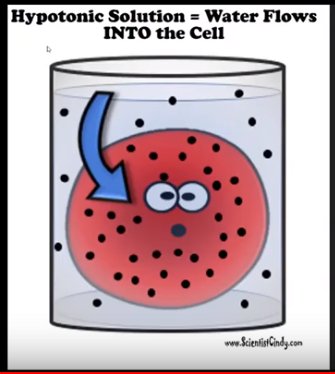
- This could be a negative value because your Bag (cell) is losing water (Hypertonic).
- This could be a positive value because your Bag (cell) is gaining water (Hypotonic)
State whether the bag is hypertonic, isotonic, or hypotonic to the beaker.
- If it loses weight = Hypertonic
- If it gains weight = Hypotonic
- If there is no weight change = isotonic
- Each of the bags were placed in separate beakers that contained either 10% sucrose or 30% sucrose. The bags were weighed every 15 minutes for 60 minutes. The dialysis tubing that was used is permeable to water but not to sucrose.
10% Sucrose Solution
|
30% Sucrose Solution
|
The solute (sugar) concentration on the inside of the bag in both cases was 0%. In both cases, we see that osmosis occurred. Water diffused from outside of the bag to inside of the bag. However, the rate at which osmosis occurred varied between the dialysis bag placed in 10% sucrose, as compared to the dialysis bag placed in 30% sucrose solution. The reason for this is that the CONCENTRATION GRADIENT is GREATER between the 30% sucrose outside the bag and the 0% sucrose inside the bag. Therefore, osmosis occurred more quickly in the bag placed in the 30% sucrose solution!
Isotonic Solution
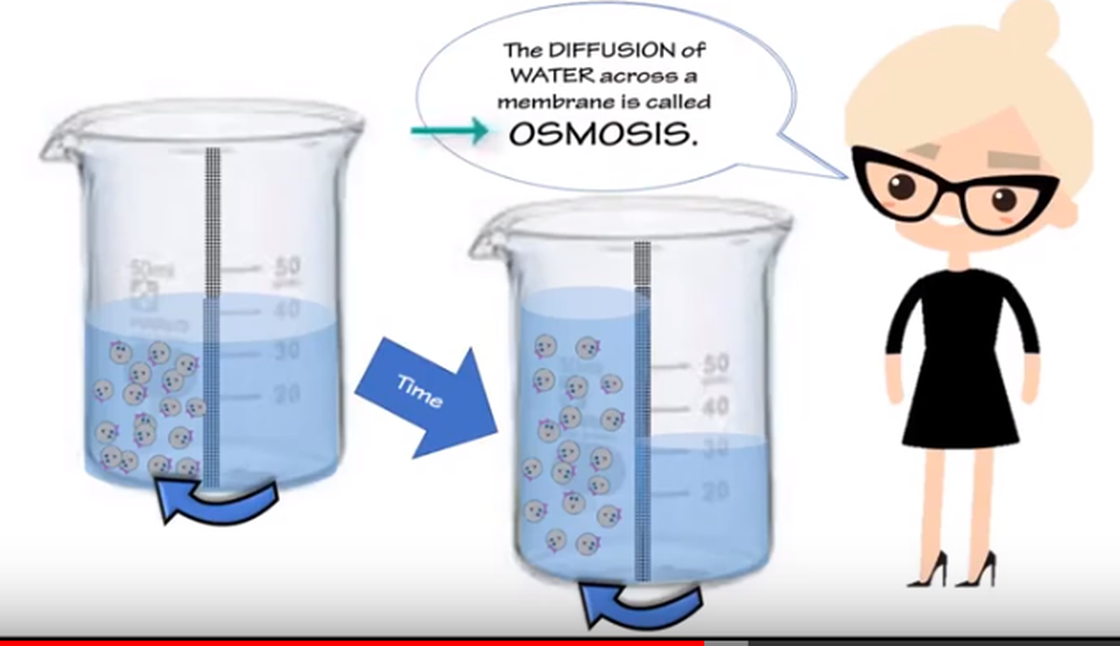
Substances will always go from higher concentration to lower concentration. This is diffusion. When the substance diffusing is WATER, we call this osmosis.
DEFINITIONS
DEFINITIONS
- Solution: A homogenous liquid mixture of two or more substances.
- Diffusion: The passive movement of matter; the spontaneous tendency of a substance to move down its concentration, pressure, or temperature gradient. Matter essentially moves (or diffuses) from an area of higher to lower concentration.
- Selectively permeable membrane: Some substances can cross the membrane while others cannot. Several factors contribute to this selectable (pore size, electric charge, etc.). The cell membrane is a severely permeable membrane, and therefore it regulates the passage of substances into and out of the cell. Osmosis: The diffusion of water (or some other solvent) across a selective permeable membrane; water diffuses to a hypertonic area. When water enters or leaves the cell it is usually accomplished by osmosis.
- Dialysis: The diffusion of a solute across a selectively permeable membrane. Dialysis can be used to separate a mixture of dissolved substances on the bases of their size.
- Concentration Gradient: The difference between the higher concentration, pressure, or temperature and the lower concentration, pressure, or temperature is referred to as the gradient.
- Hydrophobic molecules – Are lipid soluble and can pass through the membrane rapidly
- Polar molecules – Do not cross the membrane rapidly
- Osmosis - is the diffusion of a liquid solvent (water) through a selectively permeable membrane.