ENERGY
The subatomic particles that make up the atoms that make up ALL MATTER, including you and I, and infused with KINETIC ENERGY! That means that the subatomic particles are in constant motion.
|
It is pretty astonishing, but actually it makes a lot of sense! You see, we know that ALL atoms are composed of 99.999999999999999% EMPTY SPACE! But how can that be? Why don't we simply fall through the chairs we sit on or fall into the floor?
|
E=mc2
Einstein's famous equation is so simple, yet totally profound! Einstein's simple equation not only quantified the relationship between energy and mass, but he even showed that energy and mass were, in fact, simply 2 forms of the same thing! Einstein speaking about the equation E = mc2 (211kB, .MP3 file) From the soundtrack of the film Atomic Physics. Copyright © J. Arthur Rank Organisation, Ltd., 1948.
|
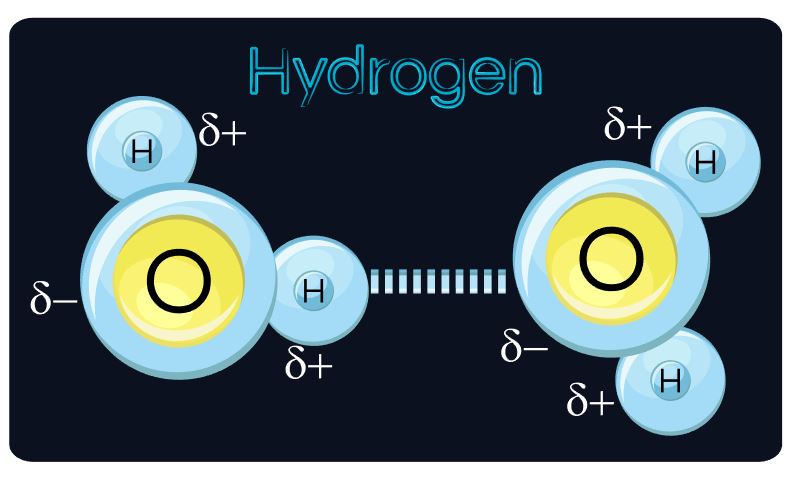
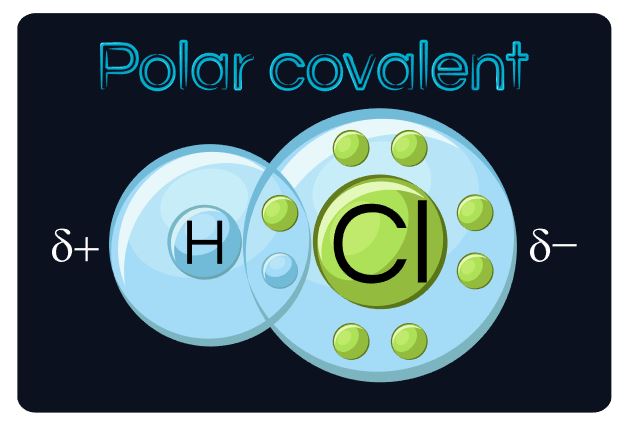
The electrostatic repulsion that exists between the outer negatively-charged electrons of one object or person and another object or person, prevent atoms from being able to "pass through" one another. Even on a small scale, individual atoms can't "pass through" each other either. At best, a chemical reaction can occur and a chemical bond may form from an interaction between the outermost electrons of each atom.
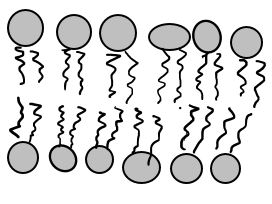
Diffusion
Before we talk about osmosis, we must first understand diffusion. The word diffusion comes from the Latin word for "spreads out". In nature, molecule will behave in such a way to "spread out" from an area of high concentration to an area of low concentration, until a time in which those concentration become equal. At this state, the substance is said to be at equilibrium. it is this phenomenon that is responsible for the air currents and ocean currents, as well as the diffusion of a substance in liquid. Since our cells are in an aqueous environment, we will be looking at this type of diffusion.
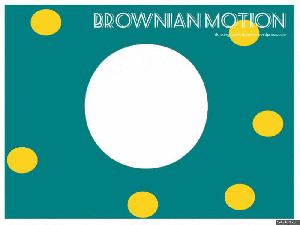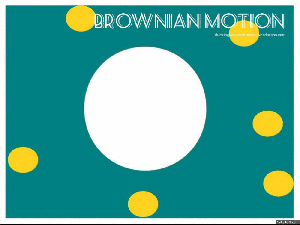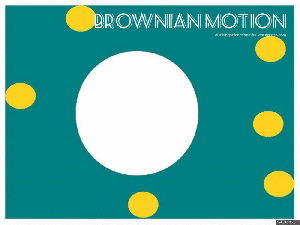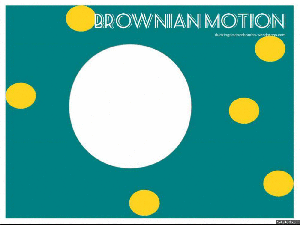
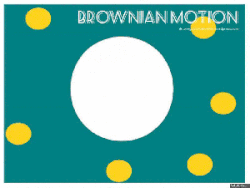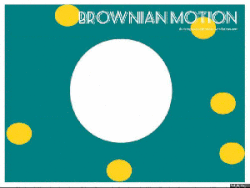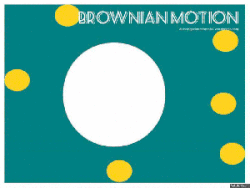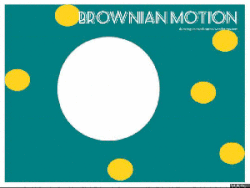
Gasses and liquids behave this way because their atoms are always in motion. This motion is called "Brownian motion". Brownian motion causes molecules to collide with each other which makes them spread out to maximize their area to move in.They want some"elbow room"!
Gasses and liquids behave this way because their atoms are always in motion. This motion is called "Brownian motion". Brownian motion causes molecules to collide with each other which makes them spread out to maximize their area to move in.They want some"elbow room"!
CELL TRANSPORT
DIFFUSION
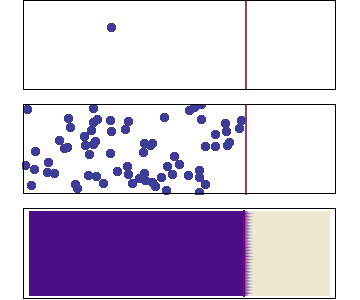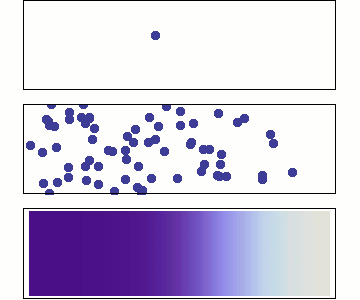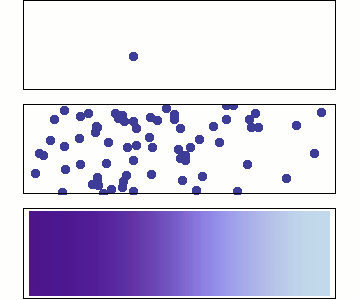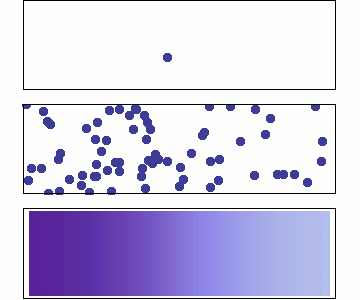 Diffusion occurs because molecules and atoms are always in motion.
Diffusion occurs because molecules and atoms are always in motion.
Before we talk about osmosis, we must first understand diffusion. The word diffusion comes from the Latin word for "spreads out". In nature, molecule will behave in such a way to "spread out" from an area of high concentration to an area of low concentration, until a time in which those concentration become equal. At this state, the substance is said to be at equilibrium. it is this phenomenon that is responsible for the air currents and ocean currents, as well as the diffusion of a substance in liquid. Since our cells are in an aqueous environment, we will be looking at this type of diffusion.
Gasses and liquids behave this way because their atoms are always in motion. This motion is called "Brownian motion". Brownian motion causes molecules to collide with each other which makes them spread out to maximize their area to move in. They want some"elbow room"!
Gasses and liquids behave this way because their atoms are always in motion. This motion is called "Brownian motion". Brownian motion causes molecules to collide with each other which makes them spread out to maximize their area to move in. They want some"elbow room"!
2
After some time, that pee will then spread out evenly over the volume of that pool reaching the unsuspecting pool-dwellers at the far end of the pool.
This is because in diffusion, liquids (or gasses) flow from an area of high concentration to an area of low concentration, until an equilibrium (no difference in concentration) is achieved. In other words, the pee is equally distributed everywhere.
This is because in diffusion, liquids (or gasses) flow from an area of high concentration to an area of low concentration, until an equilibrium (no difference in concentration) is achieved. In other words, the pee is equally distributed everywhere.
OSMOSIS
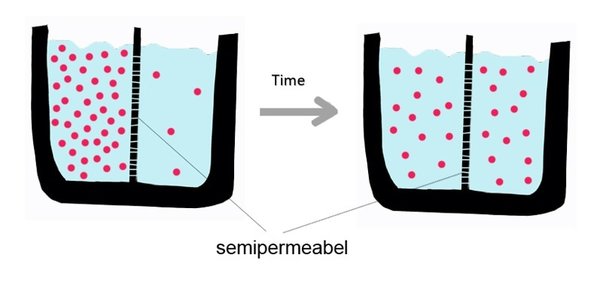
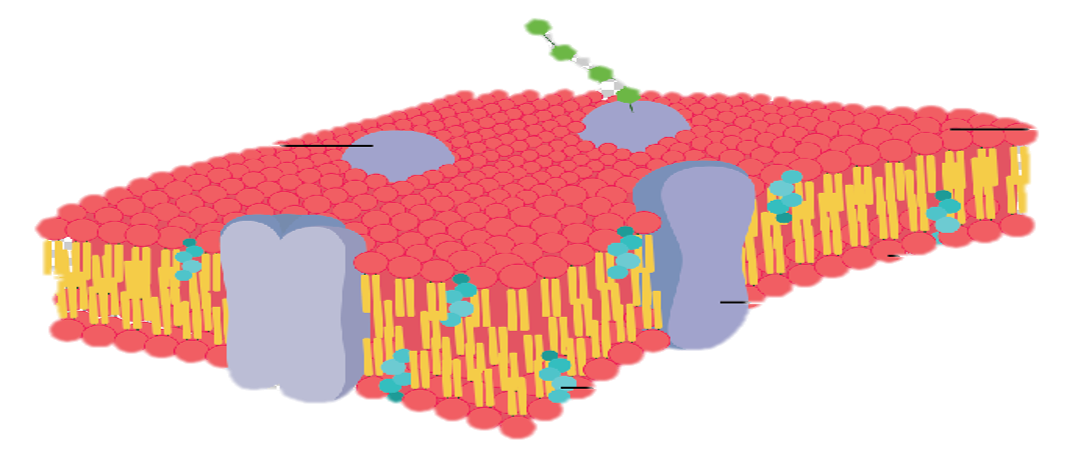
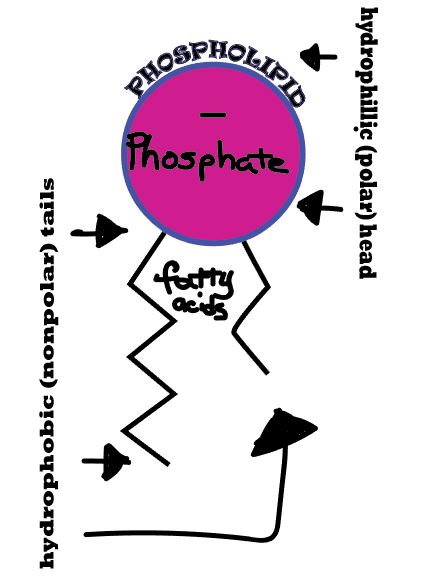
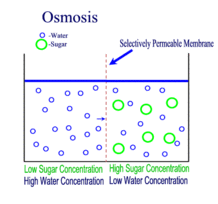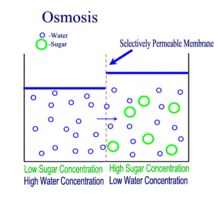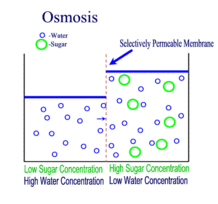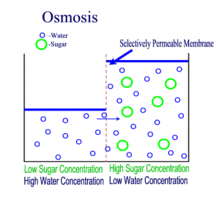
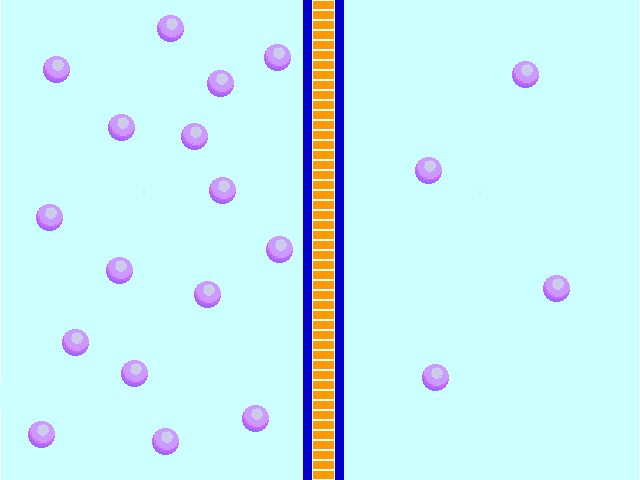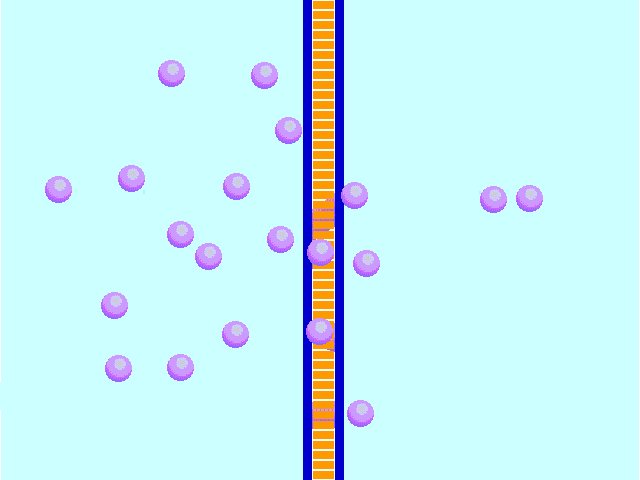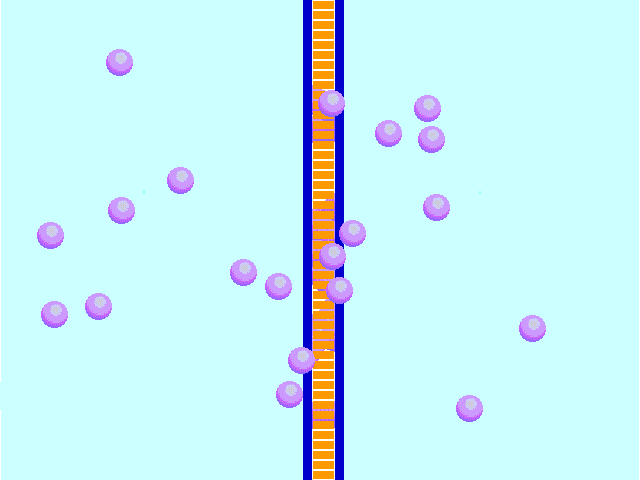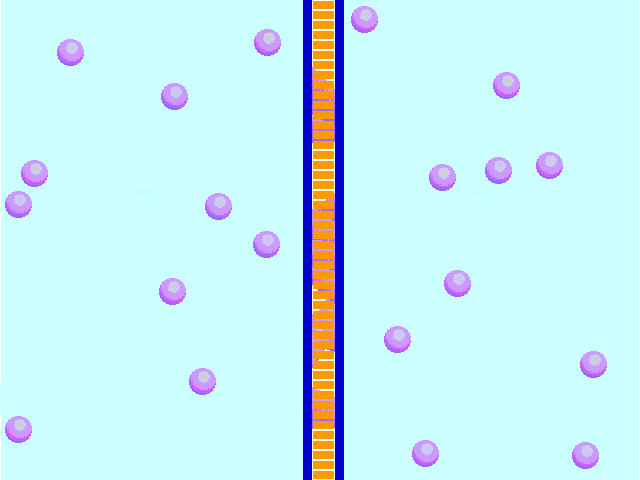
Diffusion behaves in the way just described, because it is able to move freely and unhindered. In biological systems, molecules are not permitted to move freely. The movement of ions and molecules is tightly regulated by the cell membrane. The cell membrane is selectively-permeable . Substances are permitted to pass through under certain conditions through pores or channels or transporters. The movement of water across the membrane is of particular importance to biological systems. Failure of the cell to maintain osmotic balance with its environment can have a catastrophic negative effects. The movement of water molecules across the semi-permeable cell membrane is called osmosis.
In osmosis, we only concern ourselves with the movement of water molecules (H2O) across the cell membrane. As we saw in diffusion, molecules will travel from an area of high concentration to an area of low concentration.
To understand this concept, it is important to think about a solution in terms of its components.
- The liquid substance that acts to dissolve particles, is called the solvent.
- The particles that get dissolved are called the solute.
- Hypotonic - a hypotonic solution has a LOWER concentration of solutes than are found in the intracellular fluid
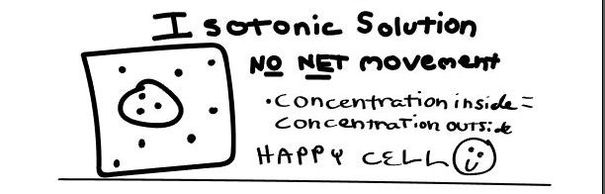
- Isotobic - an isotonic solution is one that has the SAME concentration of solutes that are found in the intracellular fluid
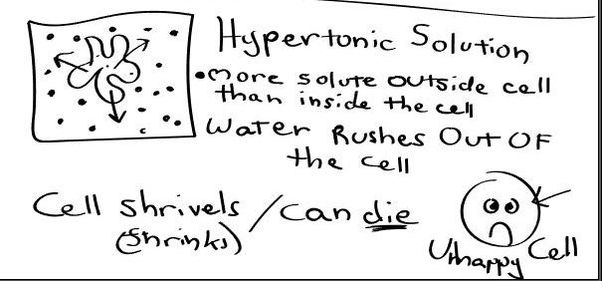
- Hypertonic - a hypertonic solution has a HIGHER concentration of solutes than are found in the intracellular fluid
|
In biological systems, the solute in unable to freely diffuse across the cell membrane, so it builds up a concentration gradient. This means that there is a higher concentration of solute on one side of the membrane than there is on the other.
The water molecules, on the other hand, CAN move freely across the cell membrane and will travel from an area of high concentration to low concentration. This traveling from high concentration to low concentration can be termed as travelling down its concentration gradient. |
|
|
|
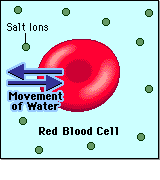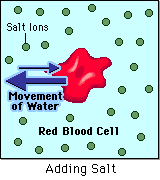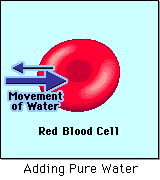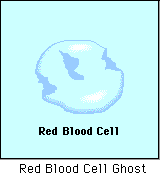
HYPOTONIC SOLUTION
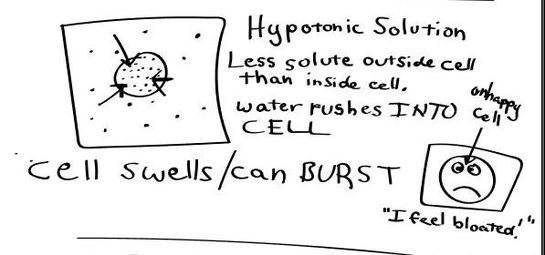
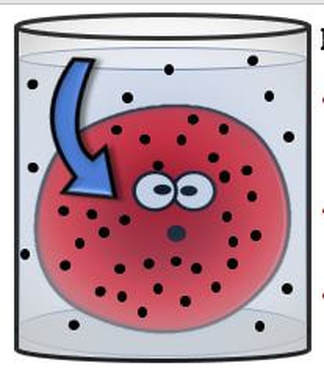
A hypotonic solution is one that has "less" concentration of solute to solvent than the intracellular fluid (fluid inside of the cell). When a cell is placed in a hypotonic solution, water rushed INTO the cell and causes it to SWELL or BURST.
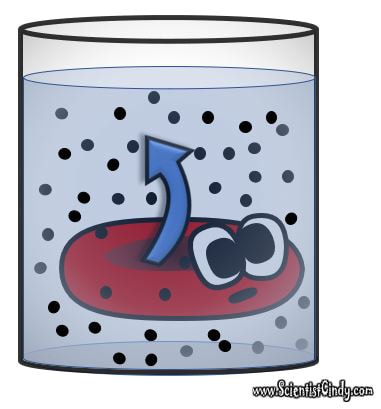
HYPERTONIC SOLUTION
|
|
|