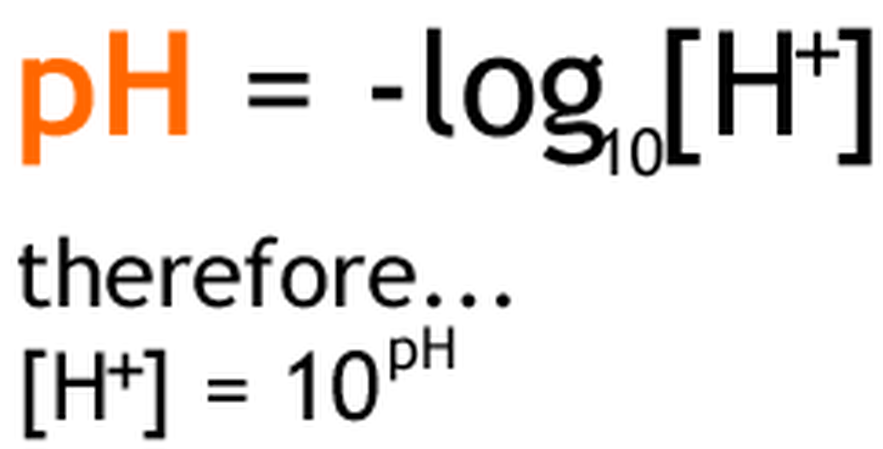Blood. Human blood contains a buffer of carbonic acid (H 2CO 3) and bicarbonate anion (HCO 3 -) in order to maintain blood pH between 7.35 and 7.45, as a value higher than 7.8 or lower than 6.8 can lead to death. In this buffer, hydronium and bicarbonate anion are in equilibrium with carbonic acid.
pH is important for Biological Processes. In liquid water, a small percentage of the water molecules dissociate or break apart into hydrogen ions (H+) and hydroxide ions (OH-). These ions are very reactive, and changes in their concentrations can drastically affect a cell’s proteins and other complex molecules.
Acids
- Some chemical compounds contribute additional H+ to an aqueous solution, whereas others remove H+ from it. A substance that donates hydrogen ions to solutions is called an acid. An example of a strong acid is hydrochloric acid (HCl), the acid in the gastric juice in your stomach. An acidic solution has a higher concentration of H+ than OH-.
Bases

- Some chemical compounds contribute additional hydroxide ions (OH-) to an aqueous solution, or remove H+ from it. These substances are considered basic or alkaline.
In the human circulatory system, red blood cells deliver oxygen and pick up unwanted carbon dioxide from the cells of the body. This carbon dioxide can combine with the water in blood plasma to form carbonic acid (H2CO3). When this occurs, the carbonic acid may then disassociate, to form bicarbonate (HCO3–) and (H+) or carbonate (CO32–) and 2(H+). These dissociated protons (H+) decrease the pH of the blood, causing the blood to become acidic.
The human body constantly monitors the internal environment for changes that could mean DANGER. Our bodies sense these changes in pH and certain physiological mechanisms are put into action that attempt to counteract the imbalance.
Some of the ways in which our bodies cope with changes in blood pH are to
Some of the ways in which our bodies cope with changes in blood pH are to
- increase or decrease the rate of breathing to adjust the amount of carbon dioxide entering the blood stream from the lungs.
- adjust the rate or amount of bicarbonate reabsorption occuring in the kidneys.
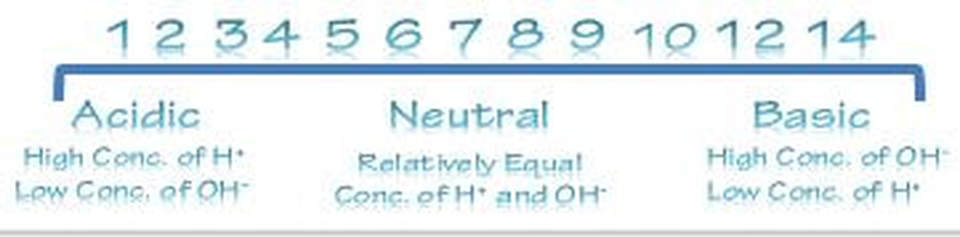
pH Scale
- We use the pH scale to describe how acidic or basic a solution is (pH stands for potential of hydrogen).
- The pH scale measures how acidic or basic a substance is. It ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic, and a pH greater than 7 is basic.
- pH Scale
- Each pH unit represents a 10-fold change in the concentration of H+ in a solution. For example, lemon juice at pH 2 has 10 times more H+ than an equal amount of a cola at pH 3 and 100 times more H+ than tomato juice at pH 4.