Biochemical Reactions
What is an "organic" molecule?
The word "organic" has a very different meaning in the sciences, than it does in the grocery store. In the grocery store "organic" usually means that the food item is free of pesticides, artificial preservatives or genetic modification.
In the sciences, organic molecules are the molecules required for life! These molecules can be identified by their long chains or rings of carbon atoms which usually also contain a combination of hydrogen, oxygen, and nitrogen.
The word "organic" has a very different meaning in the sciences, than it does in the grocery store. In the grocery store "organic" usually means that the food item is free of pesticides, artificial preservatives or genetic modification.
In the sciences, organic molecules are the molecules required for life! These molecules can be identified by their long chains or rings of carbon atoms which usually also contain a combination of hydrogen, oxygen, and nitrogen.
|
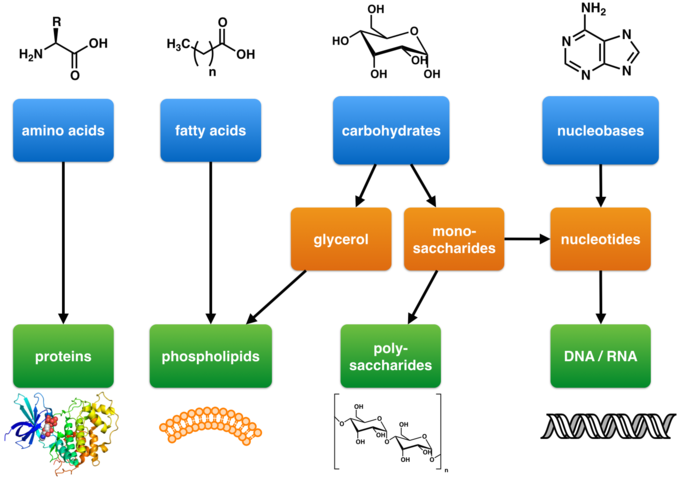
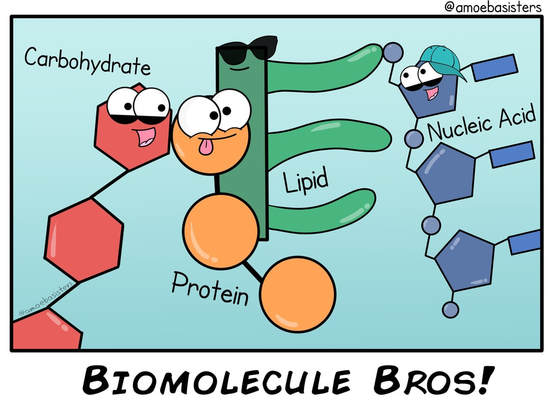
The four major classes of biological macromolecules are
Assembling larger molecules from smaller molecules is a common theme in microbiology. These types of reactions are categorized as SYNTHESIS reactions.
|
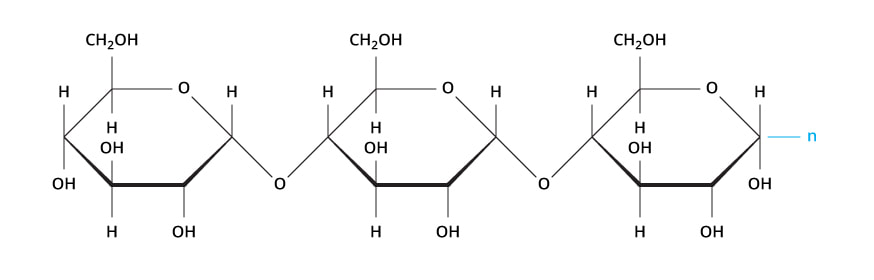
Chemical Structure of Carbohydrates
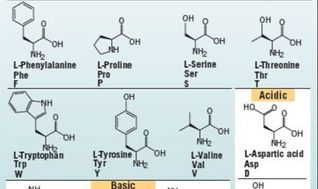
Chemical Structure of Amino Acids
(forms proteins) |

One of the more common ways for a synthesis reaction to occur is a dehydration or condensation synthesis reaction. In these types of reactions, a hydroxyl group (OH-) and a hydrogen (H+) will be removed from the subunits and water is released as a byproducts. As this occurs, a strong covalent bond is formed.
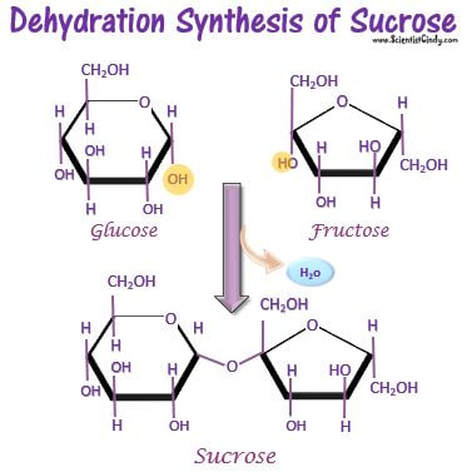
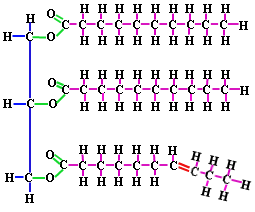
For example, in a dehydration synthesis reaction, or more simply "a dehydration reaction", the 2 monosaccharides, glucose and fructose, bind forming the disaccharide, sucrose and 1 water molecule is produced as a byproduct. Sucrose is what we think of a table sugar, that we would purchase as the grocery store.
Many biologically-relevant macromolecules are polymers. Polymers are long strings composed of repeated singular units called monomers. The terms monomer, dimer and polymer are general terms that can be applied to any units that can undergo polymerization.
Proteins are Polymers of Amino Acids
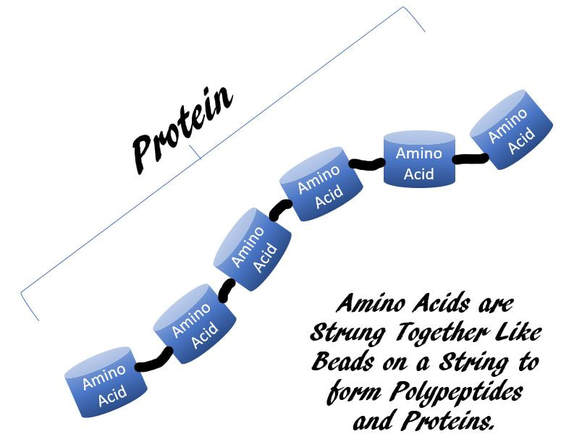
For example., amino acids are monomers. When 2 amino acids bind to each other, it is considered a dimer, or more specifically, a dipeptide. When more amino acids bind, it becomes a polymer, meaning "many". If we wanted to be specific, we should call this small string of amino acids, a polypeptide. When this string of amino acids becomes large, we call it a protein.
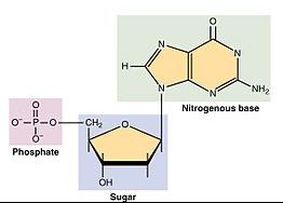
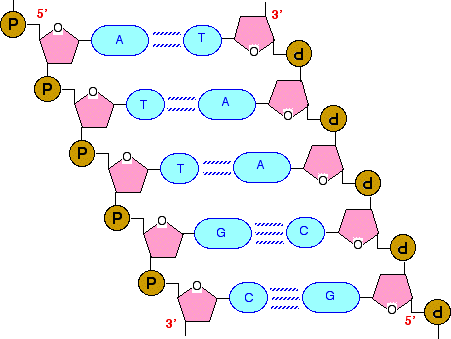
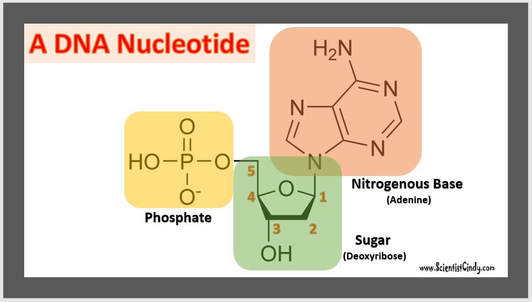
DNA and RNA are Polymers of Nucleic Acids
Examples of these monomers and polymers can be found in the sugar you might put in your coffee or tea. Regular table sugar is the disaccharide sucrose (a polymer), which is composed of the monosaccharides fructose and glucose (which are monomers). If we were to string many carbohydrate monomers together we could make a polysaccharide like starch. The prefixes “mono-” (one), “di-” (two),and “poly-” (many) will tell you how many of the monomers have been joined together in a molecule.
Four Classes of Biological Macromolecules
There are four major classes of biological macromolecules:
- carbohydrates
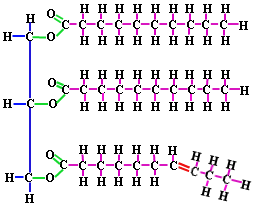
- lipids
- proteins
- nucleic acids
Each of these types of macromolecules performs a wide array of important functions within the cell; a cell cannot perform its role within the body without many different types of these crucial molecules.
Cells are immersed in an aqueous (water-like) environment. the molecules both inside and outside of cells are situated in a water-based (i.e., aqueous) environment, and all the reactions of biological systems are occurring in that same environment.
Cells are immersed in an aqueous (water-like) environment. the molecules both inside and outside of cells are situated in a water-based (i.e., aqueous) environment, and all the reactions of biological systems are occurring in that same environment.
- Carbohydrates, proteins, and nucleic acids are built from small molecular units that are connected to each other by strong covalent bonds. The small molecular units are called monomers (mono means one, or single), and they are linked together into long chains called polymers (poly means many, or multiple). Each different type of macromolecule, except lipids, is built from a different set of monomers that resemble each other in composition and size.
polymer: A relatively large molecule consisting of a chain or network of many identical or similar monomers chemically bonded to each other. - monomer: A relatively small molecule that can form covalent bonds with other molecules of this type to form a polymer.
|
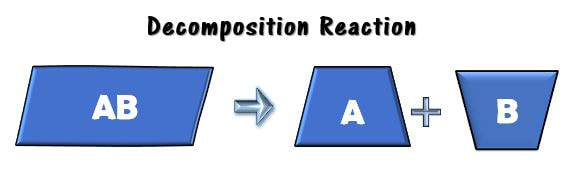
You can also break apart macromolecules into its smaller units. This may be done to release the potential energy in a chemical bond, or to re-purpose the smaller building blocks for forming other molecules. A common way that macromolecules are broken down in the body is through a DECOMPOSITION reaction.
|
, where a larger reactant is broken into smaller products. Often in biology, water is one of the reactants used to break a molecule, so it is referred to as a hydrolysis reaction (hydro = “water”; lysis = “break”): C12H22O11 + H2O → C6H12O6 + C6H12O6 Maltose Water Glucose Glucose