ORGANIC CHEMISTRY
The Chemistry of Life
THE CHEMICAL COMPOSITION OF CELLS
CLICK ON THE SLIDE SHOW BELOW TO CONTROL THE SHOW!
PAUSE, PLAY, NAVIGATE FORWARD AND BACK AND SKIP AROUND!
PAUSE, PLAY, NAVIGATE FORWARD AND BACK AND SKIP AROUND!
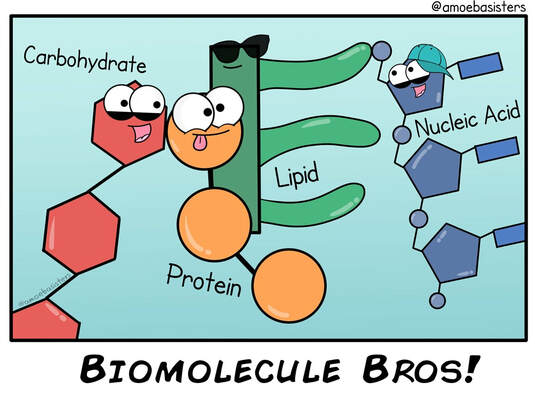
Four Classes of Biological Macromolecules
|
Introduction to Organic Chemistry
Life’s Diversity is due to being carbon-based. Most of the molecules in cell makes are made up of carbon atoms bonded to one another and to atoms of other elements. Carbon is able to form large and complex molecules, which build the structures and carry out the functions required for life. Carbon-based molecules are called organic compounds, and they usually contain hydrogen atoms as well. |
MEET CARMEN THE CARBON!!!
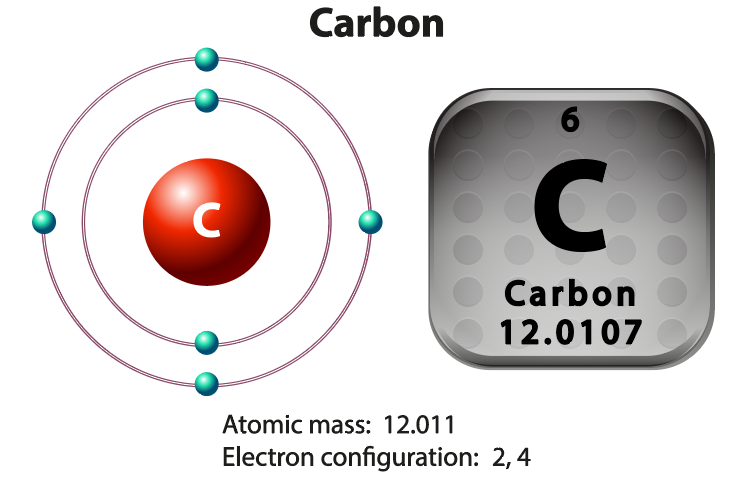
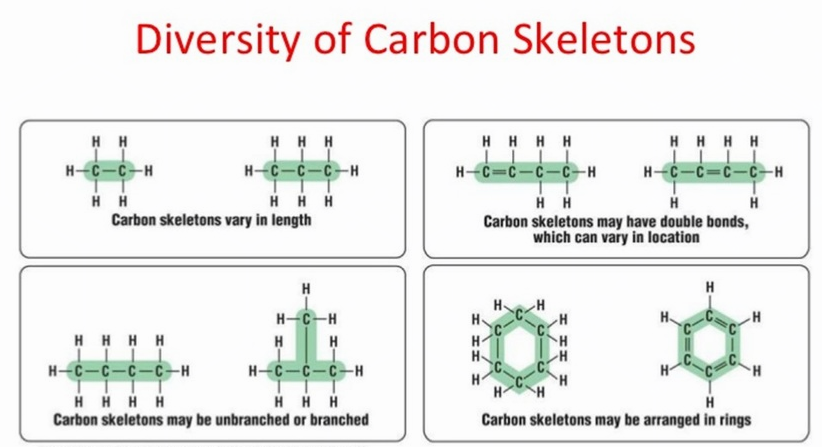
Let's first look at the unique properties of Carbon that give rise to the vast majority of molecules necessary for life. The number of electrons in the outermost shell determines an atom’s chemical properties. A carbon atom has 4 electrons in a valence shell that holds 8. Carbon completes its outer shell by sharing electrons with other atoms in four covalent bonds. This allows for a huge variety of possibilities and possible structures. This is why we observe large organic molecules with complex and elaborate shapes.
Organic compounds
are those that have carbon atoms. Remember carbon? It has 4 Valence electrons = 4 electrons in the outer shell = 4 electrons are available for bonding. Thus, carbon is able to make up to 4 strong bonds. carbon-based molecules can form a wide variety of shapes and long chains. Carbon is the element that we believe is necessary for LIFE.
Carbon Bonding
Carbon has 4 electrons available to form bonds with other atoms. This is why you will always see four lines connecting a carbon atom to other atoms. Each line represents a BOND that is formed by a pair of shared electrons (one electron from carbon and one from another atom). Each line represent a bond made with 1 valence electron from each atom participating in the bond. For example, methane (CH4) is a small organic molecule made up of one Carbon atom bound to 4 Hydrogen atoms.
are those that have carbon atoms. Remember carbon? It has 4 Valence electrons = 4 electrons in the outer shell = 4 electrons are available for bonding. Thus, carbon is able to make up to 4 strong bonds. carbon-based molecules can form a wide variety of shapes and long chains. Carbon is the element that we believe is necessary for LIFE.
Carbon Bonding
Carbon has 4 electrons available to form bonds with other atoms. This is why you will always see four lines connecting a carbon atom to other atoms. Each line represents a BOND that is formed by a pair of shared electrons (one electron from carbon and one from another atom). Each line represent a bond made with 1 valence electron from each atom participating in the bond. For example, methane (CH4) is a small organic molecule made up of one Carbon atom bound to 4 Hydrogen atoms.
Complex molecules can be formed by stringing carbon atoms together in a straight line or by connecting carbons together to form rings. The presence of nitrogen, oxygen, and other atoms adds variety to these carbon molecules.
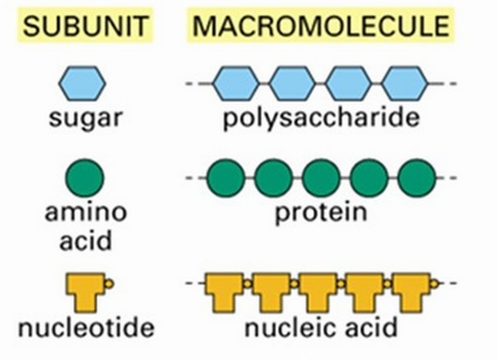
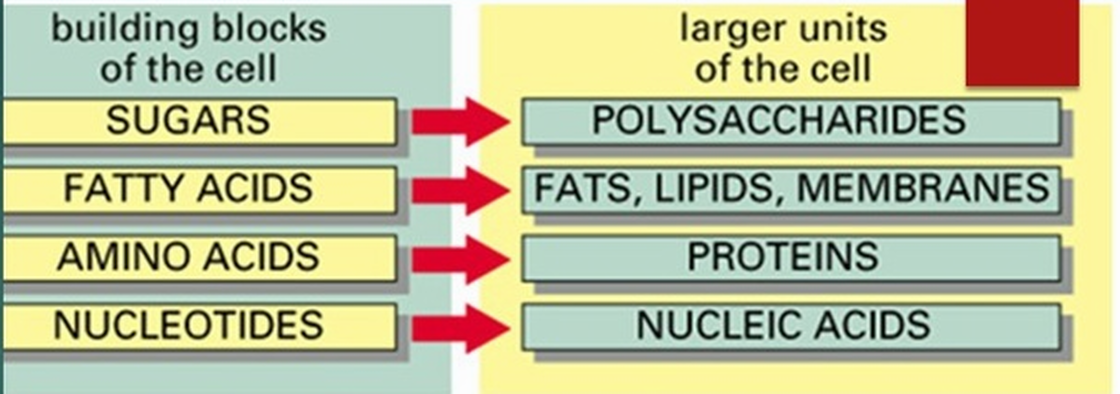
Many biologically-relevant macromolecules are polymers. Polymers are long strings composed of repeated singular units called monomers. The terms monomer, dimer and polymer are general terms that can be applied to any units that can undergo polymerization.
- Carbohydrates, proteins, and nucleic acids are built from small molecular units that are connected to each other by strong covalent bonds. The small molecular units are called monomers (mono means one, or single), and they are linked together into long chains called polymers (poly means many, or multiple). Each different type of macromolecule, except lipids, is built from a different set of monomers that resemble each other in composition and size.
polymer: A relatively large molecule consisting of a chain or network of many identical or similar monomers chemically bonded to each other. - monomer: A relatively small molecule that can form covalent bonds with other molecules of this type to form a polymer.
Carbohydrates
Sugar molecules have the formula (CH2O) n , where n is any number from 3 to 8. For glucose, n is 6, and its formula is C6H12O6. The formula for fructose is also C6H12O6, but as you can see in Figure 1, the placement of the carbon atoms is different.
Carbohydrates are classified into three groups according to the number of sugar (or saccharide) molecules present:
1) monosaccharides ( mono = 1, saccharide = sugar )
2) disaccharides ( di = 2, saccharide = sugar )
3) polysaccharides ( poly = many, saccharide = sugar )
Sugar molecules have the formula (CH2O) n , where n is any number from 3 to 8. For glucose, n is 6, and its formula is C6H12O6. The formula for fructose is also C6H12O6, but as you can see in Figure 1, the placement of the carbon atoms is different.
Carbohydrates are classified into three groups according to the number of sugar (or saccharide) molecules present:
1) monosaccharides ( mono = 1, saccharide = sugar )
2) disaccharides ( di = 2, saccharide = sugar )
3) polysaccharides ( poly = many, saccharide = sugar )
Lipids
Lipids are a class of substances that are insoluble in water. They are hydrophobic (water-fearing).
There are three major groups of lipids:
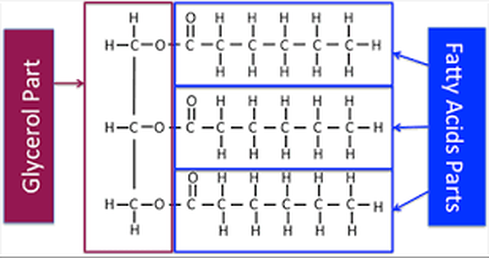
1) Triglycerides - fats, oils, and waxes.
2) Phospholipids – makes up cell membranes.
3) Steroids - hormones
Lipids are a class of substances that are insoluble in water. They are hydrophobic (water-fearing).
There are three major groups of lipids:
1) Triglycerides - fats, oils, and waxes.
2) Phospholipids – makes up cell membranes.
3) Steroids - hormones
|
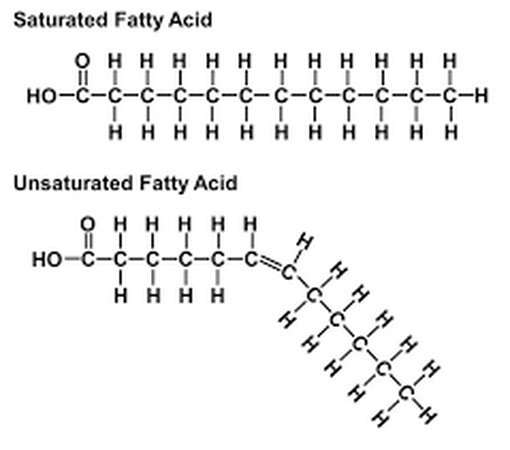
A saturated fatty acid the maximum possible number of hydrogens bonded to it. The carbon back bone contains only single bonds. Unsaturated fatty acids have less hydrogen bonded to it due to having one or more double bonds.
|
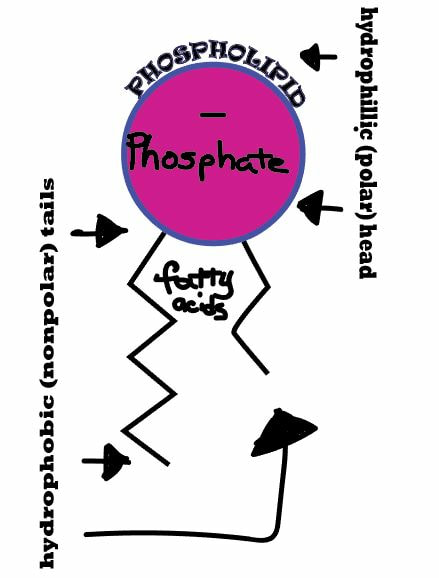
Phospholipids are the major component of cell membranes. Phospholipids are structurally similar to fats, except that they contain only two fatty acids attached to glycerol instead of three. they consist of a hydrophillic (polar) head and 2 hydrophobic (non-polar) tails. This phenomenon is called amphiphilic.
|
PROTEINS
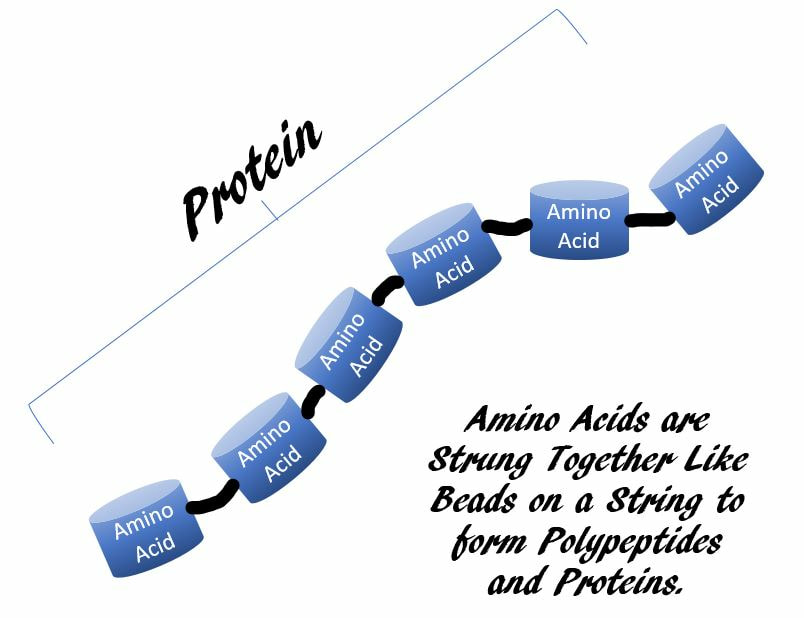
Nearly every dynamic function in your body depends on proteins.
A protein is a polymer of small building blocks called amino acids.Of all of life’s molecules, proteins are structurally and functionally the most elaborate and varied.Proteins are polymers of amino acids. Proteins perform a vast array of functions within organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another.
A protein is a polymer of small building blocks called amino acids.Of all of life’s molecules, proteins are structurally and functionally the most elaborate and varied.Proteins are polymers of amino acids. Proteins perform a vast array of functions within organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another.
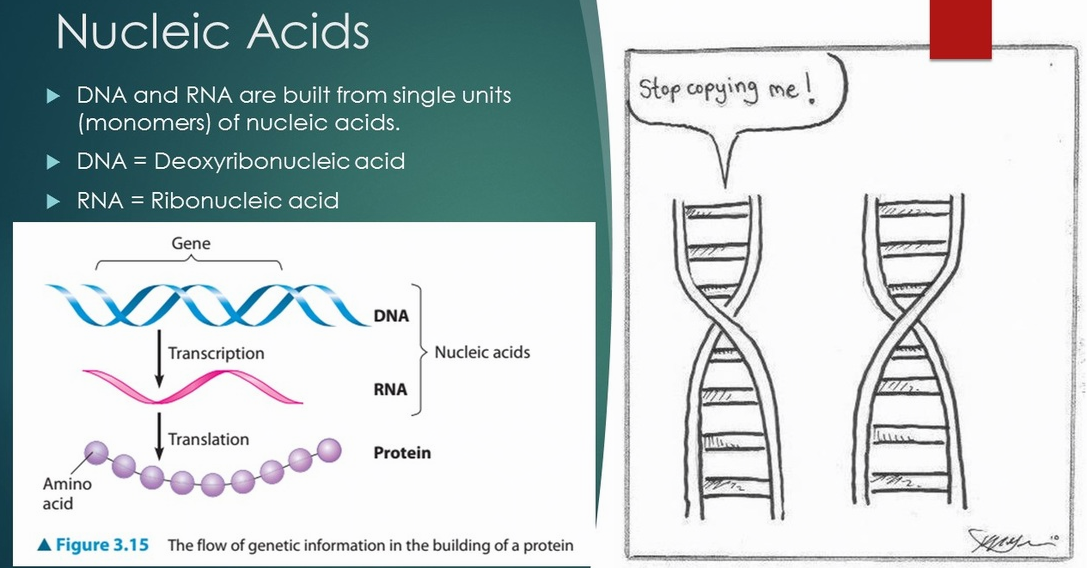
Nucleic Acids
Proteins are Polymers of Amino Acids
For example., amino acids are monomers. When 2 amino acids bind to each other, it is considered a dimer, or more specifically, a dipeptide. When more amino acids bind, it becomes a polymer, meaning "many". If we wanted to be specific, we should call this small string of amino acids, a polypeptide. When this string of amino acids becomes large, we call it a protein.
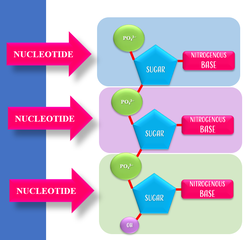
DNA and RNA are Polymers of Nucleic Acids
DNA and RNA are polymers of nucleotides.
Examples of these monomers and polymers can be found in the sugar you might put in your coffee or tea. Regular table sugar is the disaccharide sucrose (a polymer), which is composed of the monosaccharides fructose and glucose (which are monomers). If we were to string many carbohydrate monomers together we could make a polysaccharide like starch. The prefixes “mono-” (one), “di-” (two),and “poly-” (many) will tell you how many of the monomers have been joined together in a molecule.
Each of these types of macromolecules performs a wide array of important functions within the cell; a cell cannot perform its role within the body without many different types of these crucial molecules.
Cells are immersed in an aqueous (water-like) environment. the molecules both inside and outside of cells are situated in a water-based (i.e., aqueous) environment, and all the reactions of biological systems are occurring in that same environment.
For example., amino acids are monomers. When 2 amino acids bind to each other, it is considered a dimer, or more specifically, a dipeptide. When more amino acids bind, it becomes a polymer, meaning "many". If we wanted to be specific, we should call this small string of amino acids, a polypeptide. When this string of amino acids becomes large, we call it a protein.
DNA and RNA are Polymers of Nucleic Acids
DNA and RNA are polymers of nucleotides.
Examples of these monomers and polymers can be found in the sugar you might put in your coffee or tea. Regular table sugar is the disaccharide sucrose (a polymer), which is composed of the monosaccharides fructose and glucose (which are monomers). If we were to string many carbohydrate monomers together we could make a polysaccharide like starch. The prefixes “mono-” (one), “di-” (two),and “poly-” (many) will tell you how many of the monomers have been joined together in a molecule.
Each of these types of macromolecules performs a wide array of important functions within the cell; a cell cannot perform its role within the body without many different types of these crucial molecules.
Cells are immersed in an aqueous (water-like) environment. the molecules both inside and outside of cells are situated in a water-based (i.e., aqueous) environment, and all the reactions of biological systems are occurring in that same environment.