Digestion and Enzymes Lab
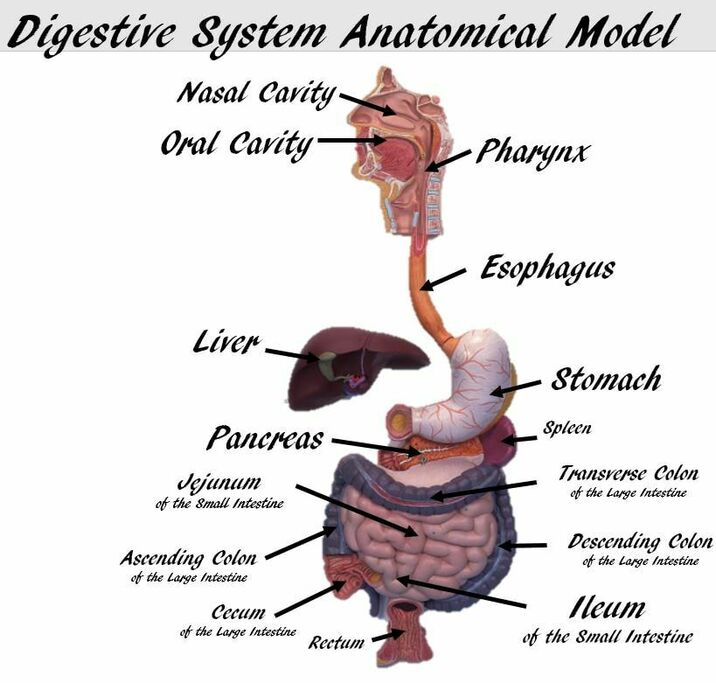
Our GI tract is a long anatomical tube where secretions of chemicals and enzymes help us safely break down foods that we ingest.
We use the GI tract to break down polysaccharides, proteins, fats, and nucleic acids into small units to prepare for absorption of these building blocks (nutrients).
Four of the enzymes have special importance in digestion of food by humans.
We use the GI tract to break down polysaccharides, proteins, fats, and nucleic acids into small units to prepare for absorption of these building blocks (nutrients).
Four of the enzymes have special importance in digestion of food by humans.
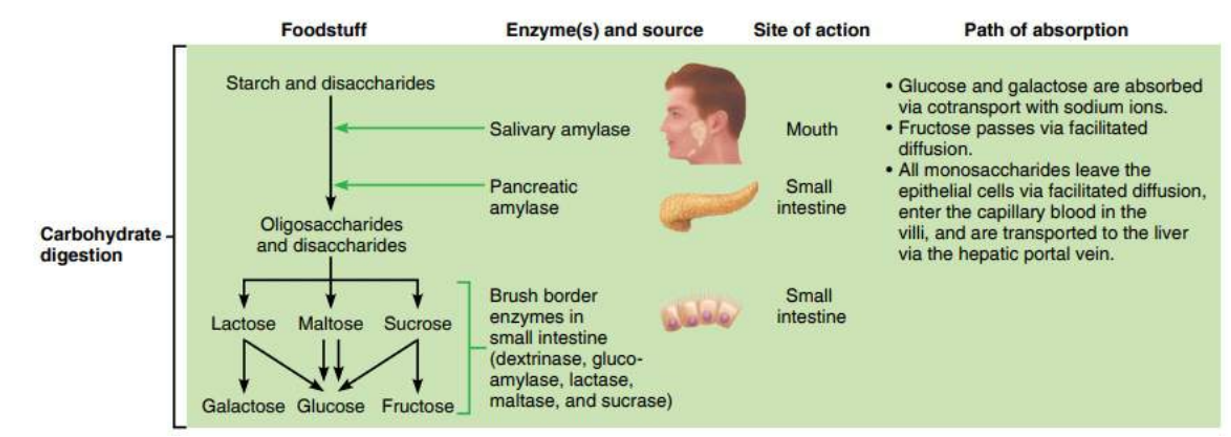
- Amylase from our salivary glands and pancreas digests starch to maltose in our mouth and small intestine.
- Lipase from the pancreas digests lipids to fatty acids and glycerol in our small intestine.
- Pepsin is a protease that begins digestion of proteins, breaking them into peptides and amino acids. Pepsinogen, is secreted by gastric glands of the stomach into the stomach. There, in the acid environment of the stomach, pepsinogen is converted into pepsin.
- Trypsin is a protease secreted into the small intestine by the pancreas. As pepsin, trypsin digests proteins into peptides and amino acids and is made and secreted in an inactive form, trypsinogen.
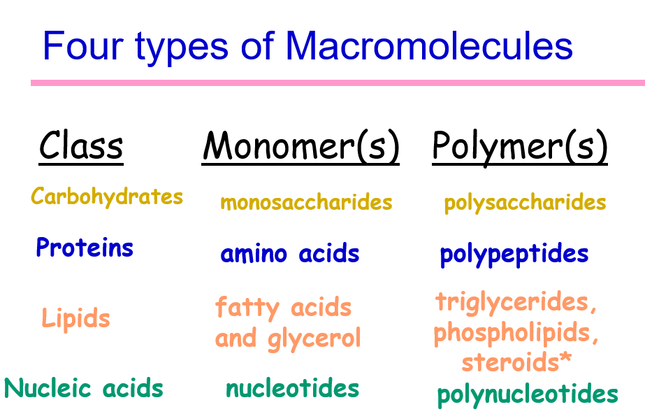
The four biological macromolecules that are important for biological functions are:
- Carbohydrates
- Proteins
- Fats
- Nucleic Acids
Enzymes
|
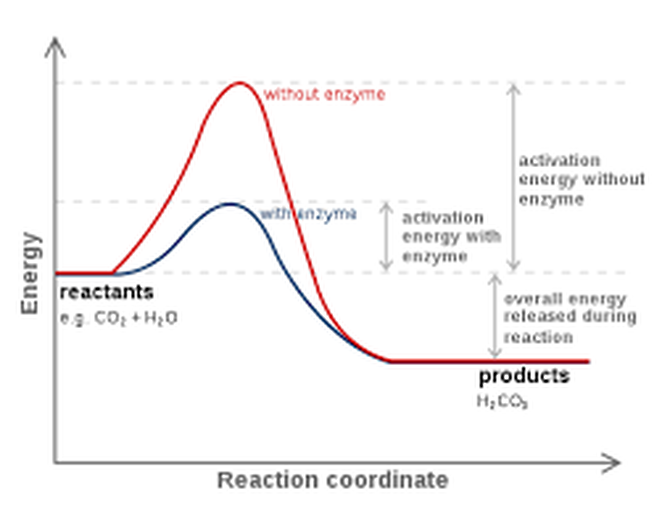
What are ENZYMES? All molecular bonds have energy barriers that must be overcome in order for the chemical reaction to occur. Enzymes cause these chemical reactions to go much faster (by several orders of magnitude) by lowering the “activation energy” of the reaction. Without enzymes, it could take decades to digest one hamburger!
|
|
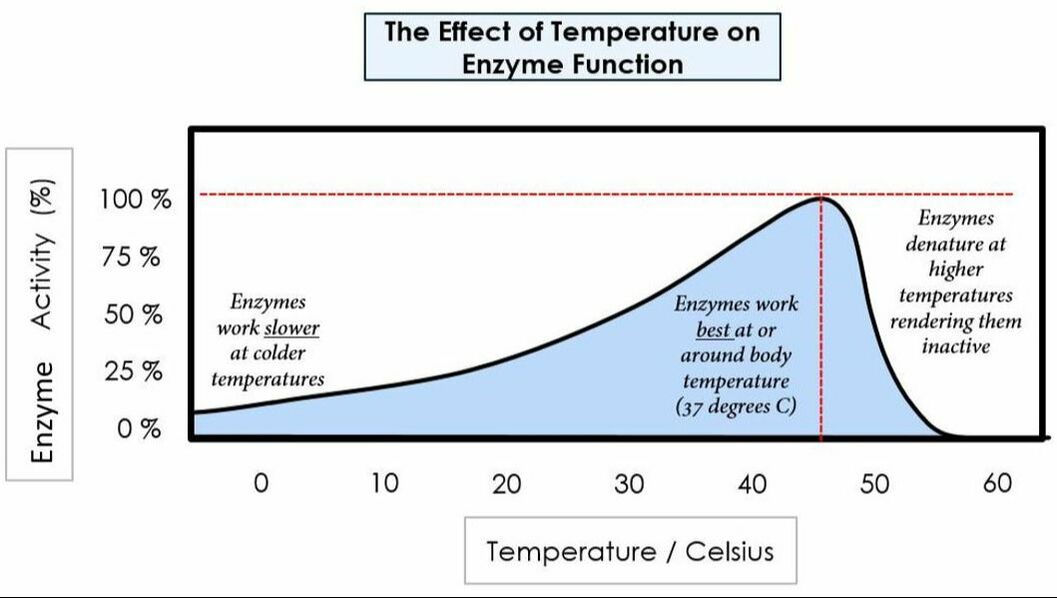
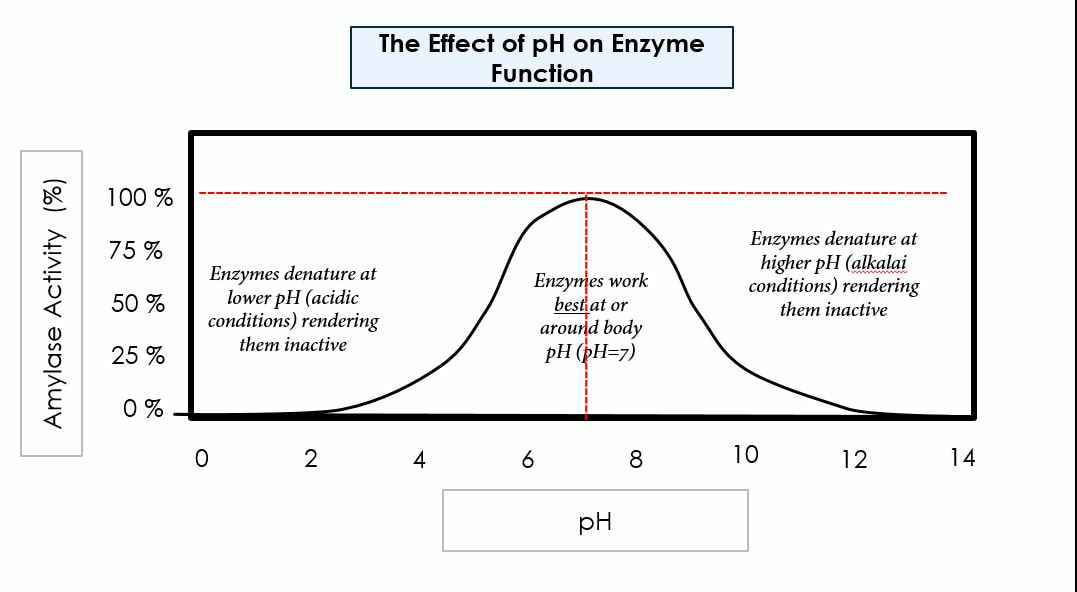
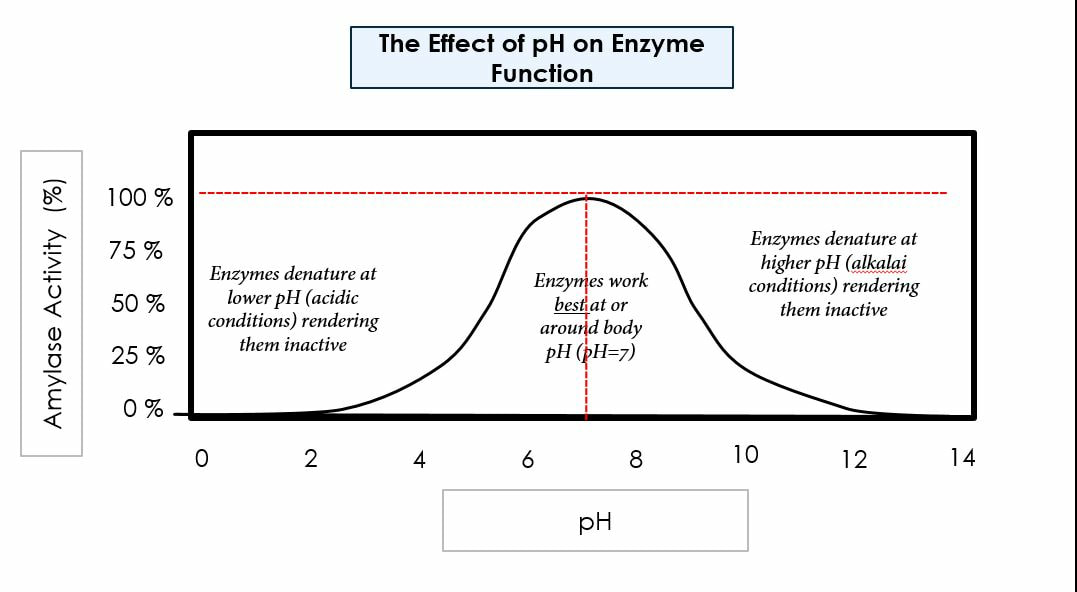
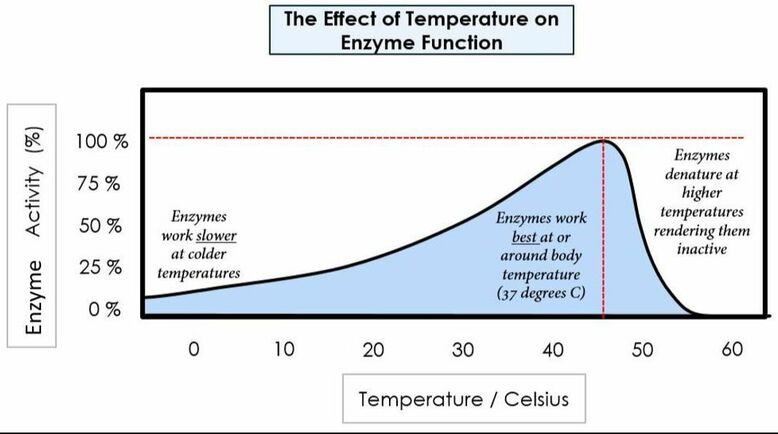
Environmental conditions affect enzymes:
Temperature
pH
We need to have a good understanding of enzymes to understand how mechanically and chemically we digest biological macromolecules. We will learn about three main enzymatic concepts: how the presence of enzymes allows us to digest foods at a faster rate, how optimal environmental factors (specifically pH and temperature) affect the rate of enzymatic digestion, how increased surface area increases chemical digestion, and how amphipathic molecules help us digest fats.
Our bodies function best in ideal conditions: ideal pH, concentration, temperature, etc. In today’s lab we will test for factors that affect carbohydrate digestion, protein digestion, and fat digestion. We will also run tests to learn how surface area affects the rate of digestion. We will use chemical indicators to test for the presence of polymers (before chemical digestion) and monomers (after chemical digestion).
Our bodies function best in ideal conditions: ideal pH, concentration, temperature, etc. In today’s lab we will test for factors that affect carbohydrate digestion, protein digestion, and fat digestion. We will also run tests to learn how surface area affects the rate of digestion. We will use chemical indicators to test for the presence of polymers (before chemical digestion) and monomers (after chemical digestion).
Carbohydrate Digestion
Lab Exercise: A. Carbohydrate Digestion in the Mouth
|
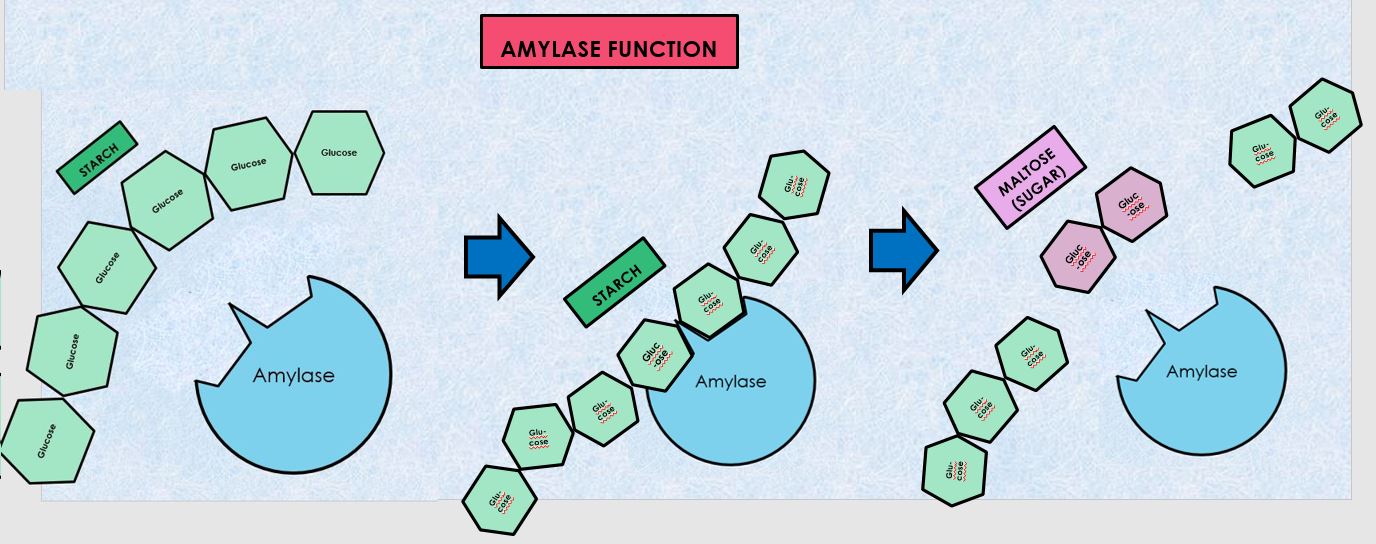
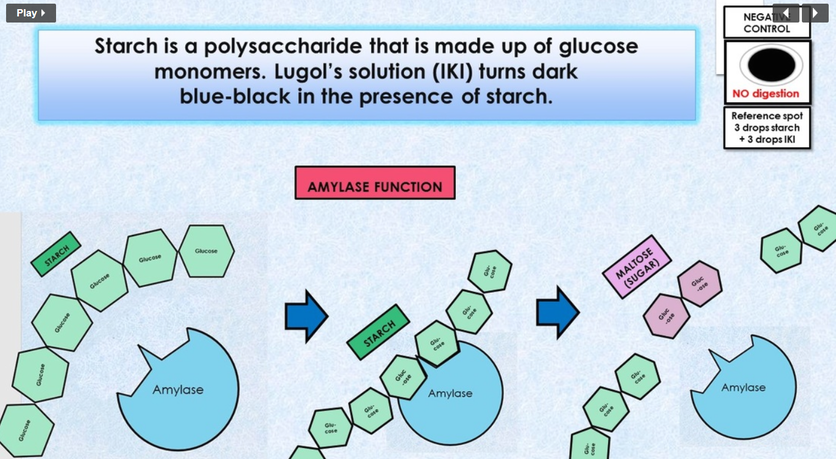
In this experiment you will investigate the hydrolysis of starch to maltose by salivary amylase. You will need to be able to identify the presence of starch and maltose, (the breakdown product of starch), to determine to what extent the enzymatic activity has occurred. Thus controls must be prepared to provide a known standard against which comparisons can be made. Starch decreases and sugar (maltose) increases as hydrolysis occurs, according to the following formula: amylase Starch + water --> maltose
Carbohydrate digestion mainly occurs in the mouth and the small intestine. AMYLASE is the enzyme responsible for breaking down polysaccharides or complex carbohydrates such as STARCH (AMYLOSE) into monosaccharides (GLUCOSE).
Amylase is found in saliva and pancreatic juice. |
When complex carbohydrates, like starch (amylose) are placed in the mouth, an enzyme called amylase which is found in the saliva begins to break down this large polysaccharide into smaller components (maltose).
Protocol
- 1. Turn on the water bath and set it to 37 ºC.
- 2. Begin to boil a beaker of water on top of the hot plate. DO NOT LEAVE THE HOT PLATE ALONE WHEN HOT. DO NOT TOUCH HOT BEAKERS.
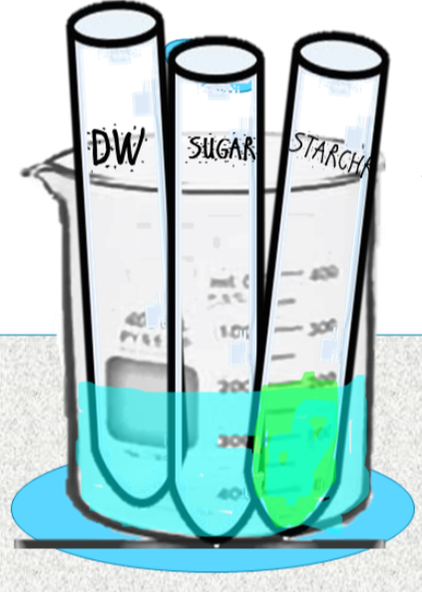
- Obtain 4 test tubes and label them with a wax pen as follows:
- W1 (negative control)
- W2 (negative control)
- S1
- S2
- 4. Add 1mL of DI water into the test tube labeled W1.
- Then add 1mL of DI water into the test tube labeled W2.
- 5. Have a volunteer add approximately 1mL of human saliva to the test tubes labeled S1 and S2 by spitting directly into the test tubes until the tube contains about 1mL of saliva.
- 6. Add 2mL of starch solution to each test tube (W1, W2, S1, S2). Stretch parafilm over the opening. Mix well.
- 7. Incubate all the test tubes in the 37 ºC water bath for 30 minutes.
- 8. Remove all the test tubes from the water bath.
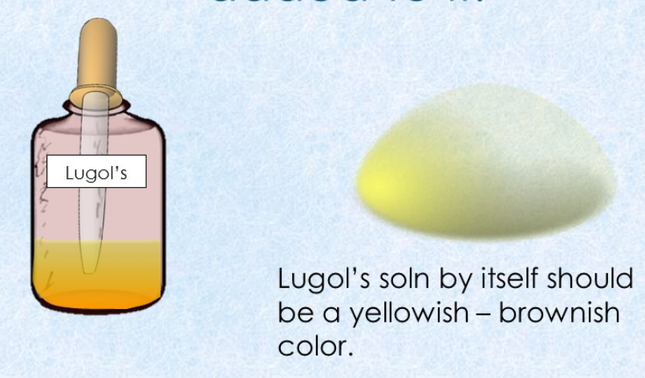
- 9. Add 5 drops of iodine indicator (IKI or Lugol's Reagent) into the test tubes labeled W1 and S1. Stretch parafilm over the opening. Mix well. Determine the Iodine reagent results.
Iodine indicator (IKI or Lugol's Reagent) detects the presence of STARCH (not glucose). Iodine indicator is yellow in color. If a solution turns...
BLACK = NO DIGESTION OCCURRED / STARCH IS STILL PRESENT
BROWN = PARTIAL DIGESTION OCCURRED / SOME STARCH IS STILL PRESENT
YELLOW/TAN = DIGESTION OCCURRED / NO STARCH IS PRESENT
BLACK = NO DIGESTION OCCURRED / STARCH IS STILL PRESENT
BROWN = PARTIAL DIGESTION OCCURRED / SOME STARCH IS STILL PRESENT
YELLOW/TAN = DIGESTION OCCURRED / NO STARCH IS PRESENT
Iodine indicator (IKI or Lugol's Reagent) RESULTS
- If starch is present, the mixture will change from its original yellowish color to a blue-black color. This color change indicates the presence of starch. his sample would be POSITIVE for STARCH, indicating COMPLETE DIGESTION.
- If starch is not present, the mixture will NOT change from its original yellowish color. This sample would be NEGATIVE for STARCH, indicating PARTIAL or INCOMPLETE DIGESTION.
- If starch is present is small quantities, the mixture will change from its original yellowish color to a brown or darker tan color. This sample would be POSITIVE for STARCH, indicating PARTIAL or INCOMPLETE DIGESTION.
|
Benedict's Reagent Test for the Presence of SIMPLE SUGARS (monosaccharides and disaccharides)
- 13. Determine if salivary amylase digested starch. How effect was saliva as digesting starch: was it complete or incomplete digestion based on the color? HINT: DEEP ORANGE OR RED WOULD INDICATE COMPLETE DIGESTION!
- 14. Clean-up: Dispose of parafilm in regular trash. Dispose of all liquids in the chemical waste container near a sink. Place the test tubes in the test tube collection bucket. Rinse and wash all serological pipets and graduated cylinders. Turn off and unplug your hot plate.
Protein Digestion
B. Protein (Albumin) Digestion in the Stomach
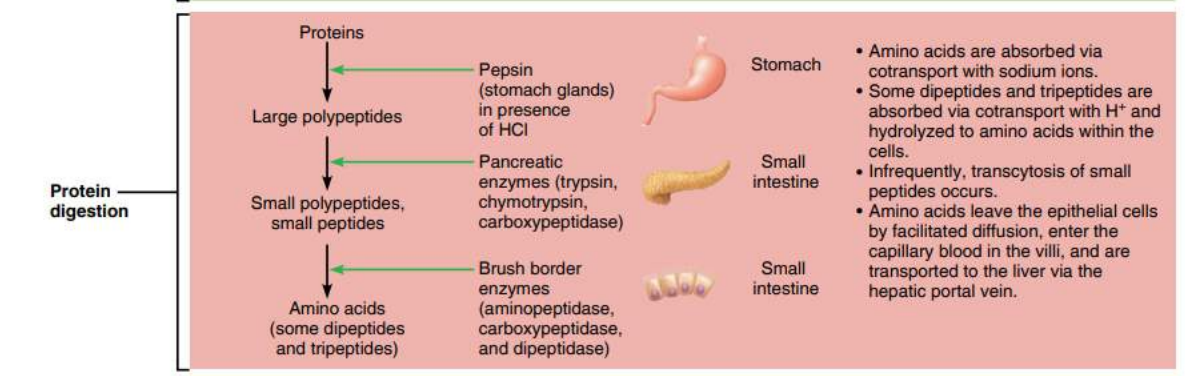
Protein Digestion is a Two-Step Process
STEP ONE: Break peptide bonds of proteins to yield smaller polypeptides. HCL in stomach first denatures the proteins to enhance chemical digestion by exposing peptide bonds.
Enzymes break peptide bonds between amino acids of proteins to make smaller polypeptides
STEP TWO: break small polypeptides into single amino acids.
Enzymes:
STEP ONE: Break peptide bonds of proteins to yield smaller polypeptides. HCL in stomach first denatures the proteins to enhance chemical digestion by exposing peptide bonds.
Enzymes break peptide bonds between amino acids of proteins to make smaller polypeptides
- In stomach: pepsin (from pepsinogen from the stomach’s chief cells)
- In intestine: pancreatic enzymes (trypsin, elastase, chymotrypsin & carboxypeptidase)
STEP TWO: break small polypeptides into single amino acids.
Enzymes:
- In the small intestines peptidases break down the polypeptides into single amino acids
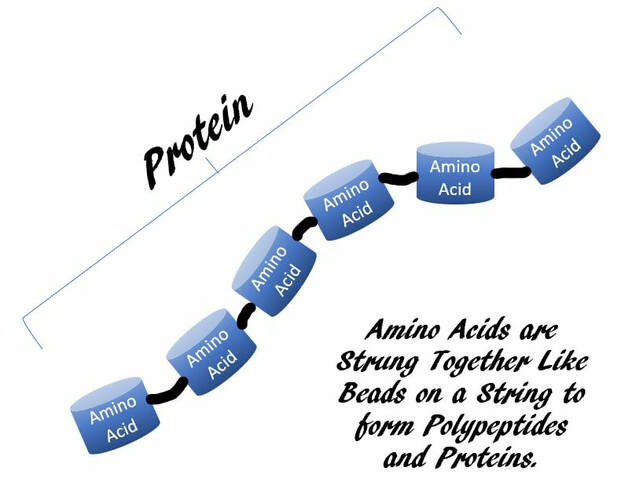
Protein digestion mainly occurs in the small intestine. Amino acids are bound together using peptide bonds to form protein (polypeptide). The enzymes responsible for breaking down protein are called proteases (for example pepsin, trypsin, and chymotrypsin), which are secreted in the stomach and small intestine. In today’s lab you will determine how temperature and pH affect the enzymatic rate of pepsin (a stomach protease). You will also determine how the presence of pepsin affects the rate of protein (albumin) digestion.
Protocol
- Obtain 5 test tubes and label them with a wax pen as follows:
- #1
- #2 (positive control)
- #3
- #4 (negative control)
- #5
- 1) In test tube #1:
- add 2mL of albumin solution
- add 3mL pepsin solution
- add 1 drop of DI water
- Stretch parafilm over the opening and mix well.
- Place test tube the 37 ºC water bath for 60 minutes while mixing the test tube every 10 minutes.
- 2) In test tube #2:
- add 2mL of albumin solution
- add 3mL pepsin solution
- add and 1 drop of 10N HCl
- Stretch parafilm over the opening and mix well.
- Place test tube the 37 ºC water bath for 60 minutes while mixing the test tube every 10 minutes.
- 3) In test tube #3:
- add 2mL of albumin solution
- add 3mL pepsin solution
- add 1 drop of 10N NaOH
- Stretch parafilm over the opening and mix well.
- Place test tube the 37 ºC water bath for 60 minutes while mixing the test tube every 10 minutes.
- 4) In test tube #4:
- add 2mL of albumin solution
- add 5mL of DI water
- add 1 drop of 10N HCl.
- Stretch parafilm over the opening and mix well.
- Place test tube the 37 ºC water bath for 60 minutes while mixing the test tube every 10 minutes.
- 5) In test tube #5:
- add 2mL of albumin solution
- add 3mL pepsin solution
- add 1 drop of 10N HCl.
- Stretch parafilm over the opening and mix well.
- Place test tube the ICE BATH for 60 minutes while mixing the test tube every 10 minutes.
- 6) Remove all the test tubes from the 37 ºC water bath and the ice bath.
- 7) Add 10 drops of Biuret reagent into each test. Stretch parafilm over the opening. Mix well.
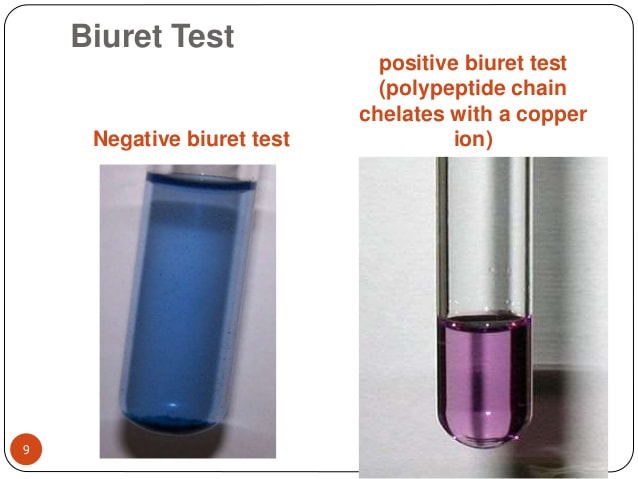
- 8) Determine the Biuret reagent results Compare each test tube to the positive control and negative control. Think about the ideal environment found in the stomach. Determine how pepsin affects protein digestion. Determine how pH affects protein digestion. Determine how temperature affects protein digestion.
- 9). Clean-up: Dispose of parafilm in regular trash. Dispose of all liquids in the chemical waste container near a sink. Place the test tubes in the test tube collection bucket. Rinse and wash all serological pipets and graduated cylinders.
You will use Biuret reagent to determine the relative number to peptide bonds in the solution; a dark purple color indicates the presence of more peptide bonds and a light purple color indicates the presence of less peptide bonds.
Fat Digestion
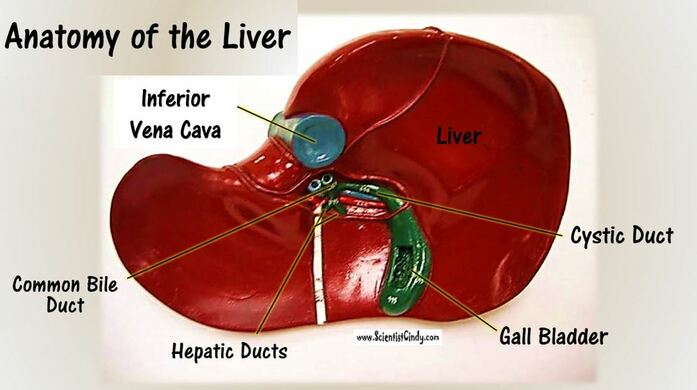
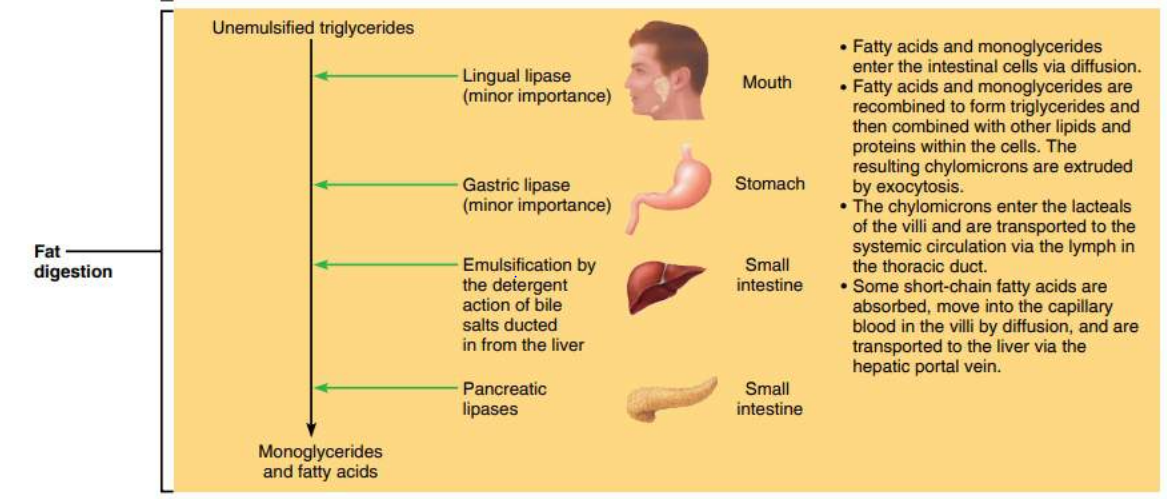
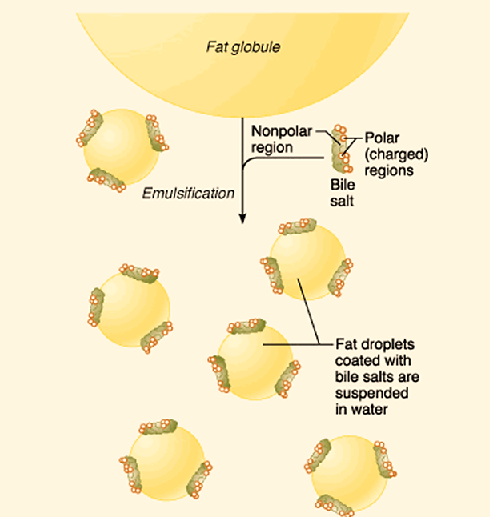
Fat digestion mainly occurs in the mouth and in the small intestine. The enzymes responsible for breaking down fats into glycerol and triglycerides are called lipases (for example salivary lipase and pancreatic lipase). The chemical that mechanically breaks down fats is called bile. The liver makes bile salts and stores them in the gall bladder. The gall bladder then secretes bile into the small intestine to aid in increasing the rate of fat digestion.
|
Goal #1: Emulsify large fat globules into tiny fat droplets. Bile salts contain the enzyme lipase. Lipase is a water soluble enzyme that cannot penetrate to the inner regions of the fat droplet. Instead, lipase will act on the surface of the fat droplets. When the larger fat globules are turned into smaller spheres, these small sphere have a higher surface-to-volume ratio, which allows lipase to effectively break down fats into its simplest components.
|
Today you will determine how quickly lipase acts with and without the presence of bile. You will use soap (an amphipathic molecule) to determine how amphipathic molecules like bile emulsify fats (heavy cream) to increase surface area.
In the next experiment you will determine how pancreatic lipase breaks down fats (heavy cream). You will then use pancreatic lipase and bile to determine how the rate of fat digestion is influenced by both bile and pancreatic lipase. You will use litmus (a pH indicator) to determine how the fats are chemically digested; alkaline solutions are blue, neutral solutions are lavender, and acidic solutions are pink.
In the next experiment you will determine how pancreatic lipase breaks down fats (heavy cream). You will then use pancreatic lipase and bile to determine how the rate of fat digestion is influenced by both bile and pancreatic lipase. You will use litmus (a pH indicator) to determine how the fats are chemically digested; alkaline solutions are blue, neutral solutions are lavender, and acidic solutions are pink.
Lab Exercise: C. Fat Emulsification & Digestion in the Small Intestine

Protocol #1: Fat Emulsion (mechanical digestion) by the molecule bile
- 1) Obtain 2 test tubes and label them with a wax pen as follows:
- soap (S)
- bile (B)
- 2) Add 20 drops of oil and 20 drops of DI water into the test tube labeled S.
- Stretch parafilm over the opening. Mix well. Record your results.
- 3) Add 2 drops of soap to the test tube labeled S.
- Stretch parafilm over the opening. Mix well. Record your results.
- 4) Add 20 drops of oil and 20 drops of DI water into the test tube labeled B.
- Stretch parafilm over the opening. Mix well. Record your results.
- 5) Add 2 drops of bile to the test tube labeled B.
- Stretch parafilm over the opening. Mix well. Record your results.
- 6) Compare your results in test tube S and test tube B.
- Explain how amphipathic molecules function to mechanically break down fats to increase surface area.
- 7) Clean-up: Dispose of parafilm in regular trash. Dispose of all liquids in the sink while running water. Place the test tubes in the test tube collection bucket. Rinse and wash all serological pipets and graduated cylinders.
Protocol #2: Mechanical and Chemical Fat Digestion in the Small Intestine
- Obtain 4 test tubes and label them with a wax pen as follows:
- #1
- #2 (positive control)
- #3
- #4 (negative control)
- 1. Add 1.5mL of Heavy Cream, 2.5mL DI water, 0.5mL bile, and 1.5mL litmus into the test tube labeled #1.
- Stretch parafilm over the opening. Mix well.
- 2) Add 1.5mL of Heavy Cream, 2.5mL pancreatic lipase, 0.5mL bile, and 1.5mL litmus into the test tube labeled #2.
- Stretch parafilm over the opening. Mix well.
- 3) Add 1.5mL of Heavy Cream, 2.5mL pancreatic lipase, 0.5mL DI water, and 1.5mL litmus into the test tube labeled #3.
- Stretch parafilm over the opening. Mix well.
- 4) Add 1.5mL of Heavy Cream, 3mL DI water and 1.5mL litmus into the test tube labeled #4.
- Stretch parafilm over the opening. Mix well.
- 5) Show your test tubes to your instructor.
- 6) Incubate all the test tubes in the 37 ºC water bath. Observe how the pH of each test tube changes every 3 minutes for 15 minutes by looking at the color change.
- 7) Remove the test tubes from the 37 ºC water bath after the incubation period.
- 8) Compare your test tube results to the positive control and negative control. Explain how bile affects the rate of chemical digestion by pancreatic lipase. Explain the role of bile and pancreatic lipase in fat digestion. Explain why the pH changes when fats are digested.
- 9) Clean-up: Dispose of parafilm in regular trash. Dispose of all liquids in the chemical waste container. Place the test tubes in the test tube collection bucket. Rinse and wash all serological pipets, graduated cylinders, and glass rods.