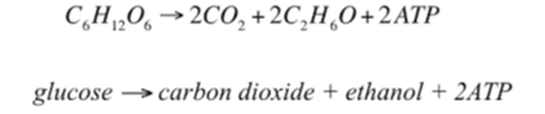|
|
|
SOLAR HEATERS
- Heat Transfer
- How is heat transferred from one source to another?
There are 3 ways in which heat can be transferred.- thermal conduction
- thermal convection
- thermal radiation
- Thermal conduction occurs when heat energy is transferred from one solid object to another through physical contact.
Heat convection occurs when bulk flow of a fluid (gas or liquid) carries heat along with the flow of matter in the fluid.
Thermal radiation is the transfer of heat energy by means of photons (light rays) or waves of electromagnetic radiation.
1) Effect of Radiation on the Growth of Organisms
What is radiation?
|
|
|
|
The factors that determine HOW damaging a particular type of radiation can be to living cells include the following:
The damage to the DNA of the cell can lead to...
|
|
Radiation is Measured in Roentgens (r). The Roentgen unit of measurement for ionizing radiation was named after Wilhelm Röntgen (1845–1923) the scientist who discovered X-rays.
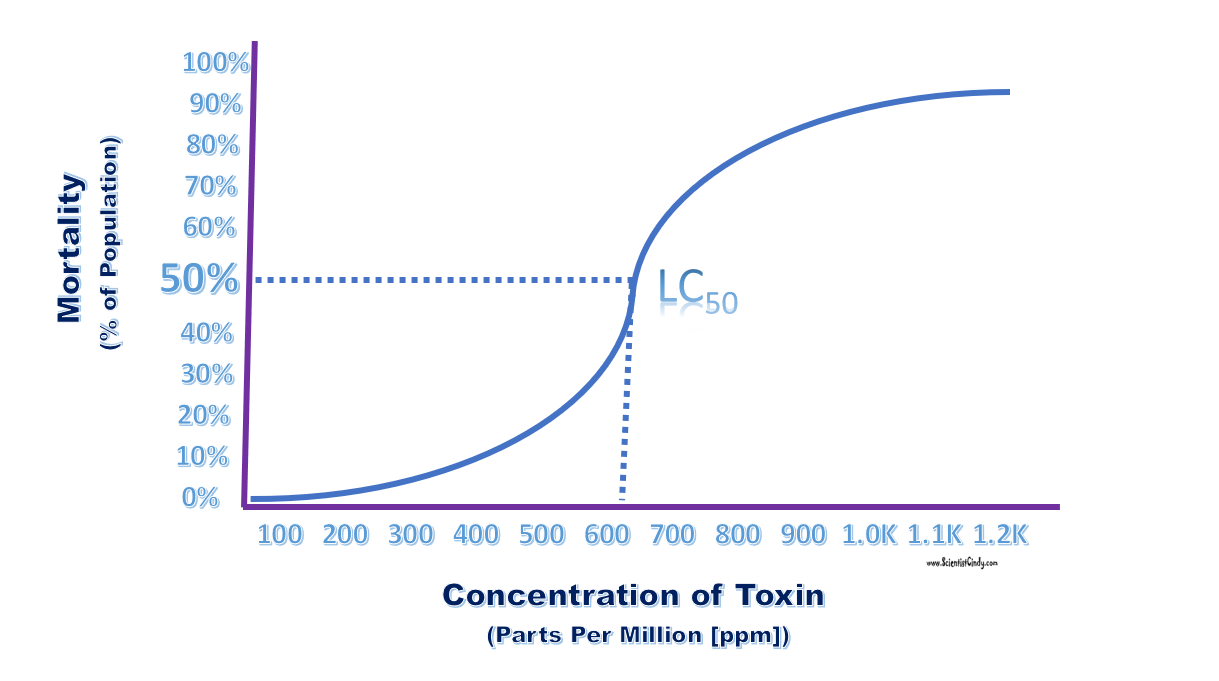
A value that is useful when analyzing and categorizing different types of ionozing radiation is LD50. LD50 (Lethal Dose 50) is the dose at which 50% of the organisms tested will die. |
|
|
|
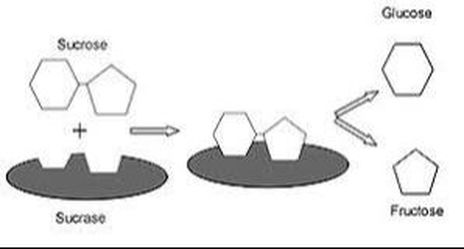
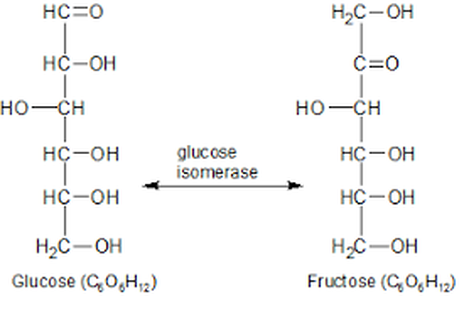
2) Fermentation and
the Production of Alcohol
Ethanol is a very clean burning fuel. This fact is easily demonstrated when ethanol is burned. When ethanol is burned, you do not observe any smoke produced!
The United States uses ethanol as a fuel additive to gasoline to reduce harmful emissions.
Soil and Groundwater Systems
 The Root Hairs of the Plant Reach into the Soil's Interstitial Spaces to Find Water
The Root Hairs of the Plant Reach into the Soil's Interstitial Spaces to Find Water
Soil and Groundwater are extremely important to plants. You may recall that the foundation of all food chains are plants. Therefore plants make up the foundation of the ecosystem.
Plants get essentially all of their water and all of their nutrients through their root systems which spread out into the soil. The properties of the soil directly affect how much water that plant will be able to access.
These properties include:
Plants get essentially all of their water and all of their nutrients through their root systems which spread out into the soil. The properties of the soil directly affect how much water that plant will be able to access.
These properties include:
- Soil particle size
- Organic content of the soil
|
|
|
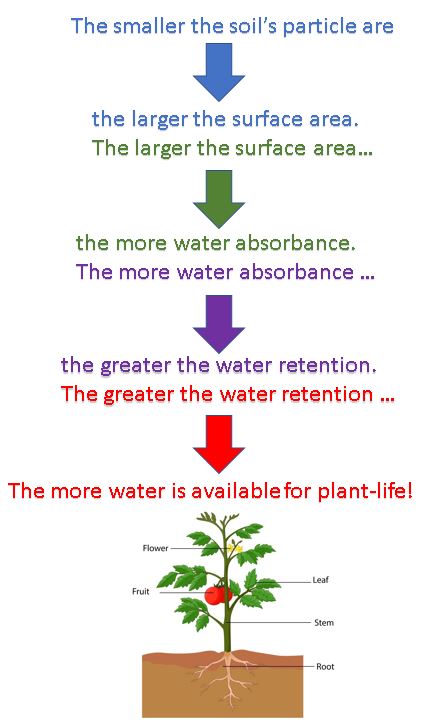
Soil Particle Size
SIZE MATTERS!
The reason why the size of the particles that make up the soil is so important to plant life, is because the the soil particle size directly influences the interstitial water content. Remember, the interstitial water content is essentially the only source of water the plant has! I say essentially, because a small amount of moisture is able to go through the plant's stomata IF humidity is very high.
Water Retention in soil science, refers to the amount of water the soil retains. The retention of water is due to the amount of available surface area of the soil's particles. Therefore, a soil's ability to retain water is strongly related to its particle size.
- Larger particles have a LOWER surface-to-volume ratio.
- Small particles have a HIGHER surface-to-volume ratio.
The amount of water retained in the soil depends on how the water droplets interact with the soil particles. As water molecules move throughout the soil (a process called percolation), the water molecules have the opportunity to absorb to (or stick to) the soil particle's surface. Smaller particles provide MORE surface area for the water droplets to absorb to. Larger particles provide less area for the water droplets to absorb to.
Soil with smaller particles allow for more water retention, which leads to healthy, happy, well-watered plants!
|
OK, cool! But, that isn't the whole story! In order for water to be retained in the interstitial spaces of soil, the water has the be able to percolate (or travel) through the soil to reach the interstitial spaces in the first place!
Groundwater moves through interstitial spaces (pores) between individual particles of soil particles. Percolation is the movement of water within the soil's interstitial spaces or pores. The larger the interstitial spaces, the higher (faster) the rate of water moves into and through the soil. |

Field Capacity is the maximum amount of water that a given soil can retain.
Wilting Point is when the soil is so dry that plants cannot get any water from it.
Absorbance in soil science is the ability of water molecules to cling (using ADHESION) to the soil particles. When a soil absorbs water, the water becomes temporarily trapped in the spaces between the soil particles.
Wilting Point is when the soil is so dry that plants cannot get any water from it.
Absorbance in soil science is the ability of water molecules to cling (using ADHESION) to the soil particles. When a soil absorbs water, the water becomes temporarily trapped in the spaces between the soil particles.
|
|
|
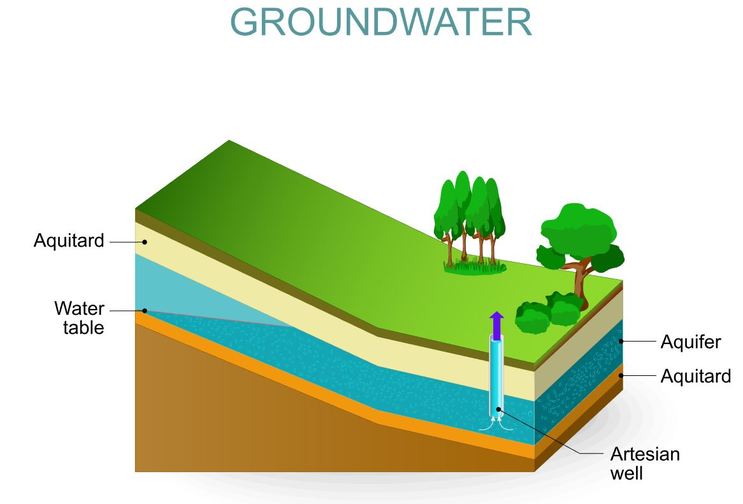
Groundwater Systems
|
Groundwater
Groundwater is the water found beneath the earth's surface, in the crevices and spaces found between particles of soil and rocks. Groundwater is stored naturally under the ground and can be accessed using wells.
|
It is important to protect our freshwater supply from toxic substances and contamination.
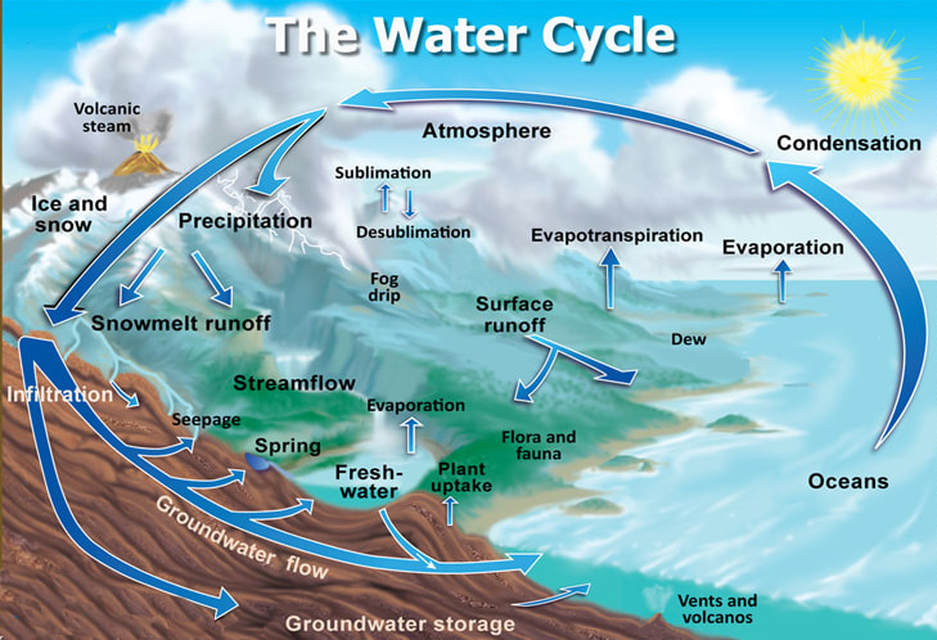
Our groundwater is vulnerable to contamination due to the water cycle. Groundwater moves very slowly and collects very slowly. Groundwater is a resource that must be conserved. In the water cycle, we see that all water comes from the ocean, and all water eventually returns to the ocean!
The Basic Steps of the Water Cycle
- Evaporation - the water from the ocean evaporates into the atmosphere
- Condensation - the water in the atmosphere condenses in clouds
- Precipitation - the water falls from the clouds as precipitation (rain, snow, hail, etc.)
- Infiltration - the water from precipitation infiltrates the soil
- Runoff - the water that is headed back to the ocean is considered runoff
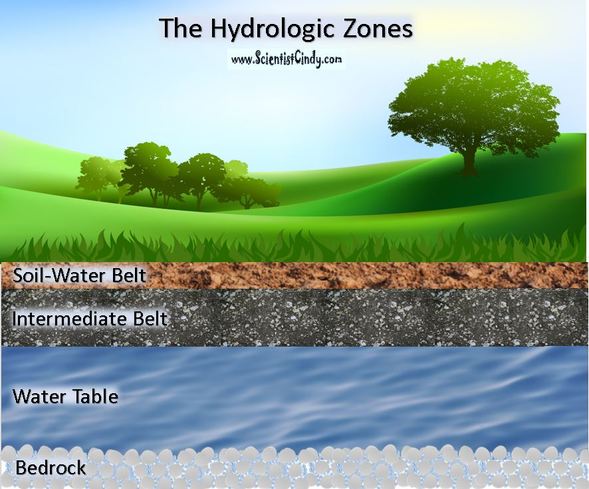
Rainfall helps replenish the soil's water supply which plants need. However, when rainfall is low, a lot of the water is lost due to evaporation and runoff or it will travel down to the water table where plants cannot reach. The shallow portion of soil that the roots of plants have access to is called the soil-water belt.
The soil-water belt can only hold a limited amount of water for a limited time. In other words, the soil-water belt has a limited holding capacity.
A sudden downpour of rain can saturate the soil-water belt and excess water will percolate downward into the intermediate belt and can continue downward to the water table.
The soil-water belt can only hold a limited amount of water for a limited time. In other words, the soil-water belt has a limited holding capacity.
A sudden downpour of rain can saturate the soil-water belt and excess water will percolate downward into the intermediate belt and can continue downward to the water table.
- LC50 is a measure of toxicity of a substance found in the environment. LC50 is commonly used to measure, monitor and quantify levels of contaminants in our water supplies or environmental toxins. The value of LC50 is usually experimentally determined by exposing a non-human sample population to the toxin. LC50 is defined as the concentration of the toxin that will kill 50% of the test population exposed to that concentration of the toxin after only a single exposure.
|
The LD50 is a similar type of measurement typically used for pharmaceuticals or consumer products not naturally found in our environment. LD50 values are typically determined experimentally by exposing a test group of animals to the substance in question. LD50 is defined as the dose that will kill 50% of the animals exposed after a single dose.
|
|
The Clean Water Act
The Clean Water Act
- The Clean Water Act was originally established in 1948 under the name Federal Water Pollution Control Act. In 1972 its name was officially changed to The Clean Water Act, along with some noted structural and policy changes. These acts were put into place in to establish water pollution regulation and oversight.
Biomagnification
|
4) Water Quality

- Copper sulfate is a contaminant that is commonly found in our water supply. This compound is used in pesticides and acts to kill bacteria, algae, roots, plants, snails, and fungi. Ingesting large amounts of this environmental contaminant can cause nausea vomiting, kidney failure and even death!
- http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/D/DDTandTrophicLevels.htmlw:en:Biomagnification

The unique properties of water include...
- Water will stop contracting as it freezes and will be expanding
- Water can become vapor at any temperature below its boiling point
- Water can diffuse into dry air through evaporation, without the need for boiling.
- High heat capacity
- Water slowly absorbs heat
- Water slowly releases heat after it is absorbed
- This keeps temperatures from getting too hot or too cold
- Temperatures are more moderate on the coast and in areas with large bodies of water.
- Areas of dry desert so extremely hot temperatures during the day and extremely cold temperatures at night, due to the lack of water in the area!
- Water is a powerful solvent. Water has been able to cut through rock to make huge canyons. It might take thousand's of years, but water is a lot tougher than it looks!
There are a lot of different measurements that can be done to determine water quality. One important test is the D.O. Test.
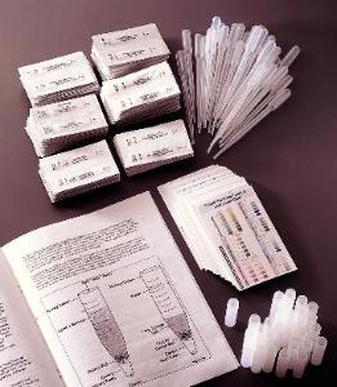
D.O. Test - The D.O. Test determines the amount of dissolved oxygen in a water sample.
- Total dissolved gas concentrations in water should not exceed 110 percent. Very high oxygen levels can lead to "gas bubble disease" which can block the flow of blood in aquatic organisms.
- Total dissolved oxygen level should not fall below 5.0 mg/L. Very low oxygen levels causes stress on aquatic organisms, since they are not getting enough oxygen to survive. Oxygen levels lower than ~ 1mg/L will result in massive loss of marine life after only a few hours.